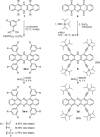
5,7,12,14-Tetrafunctionalized 6,13-Diazapentacenes
- PMID: 31609025
- PMCID: PMC7004126
- DOI: 10.1002/chem.201904516
5,7,12,14-Tetrafunctionalized 6,13-Diazapentacenes
Abstract
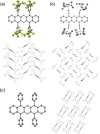
The synthesis, property evaluation, and single crystal X-ray structures of four 5,7,12,14-tetrafunctionalized diazapentacenes are presented. The synthesis of these compounds either starts from tetrabromo-N,N-dihydrodiazapentacene or from a diazapentacene tetraketone. Pd-catalyzed coupling or addition of a lithium acetylide gave the precursors that furnish, after further redox reactions, the diazapentacenes as stable crystalline materials. The performance of the tetraphenyl-substituted compound as n-channel semiconductor was evaluated in organic field effect transistors.
Keywords: X-ray diffraction; diazapentacenes; heteroacenes; tetrasubstitution.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- None
-
- Freudenberg J., Bunz U. H. F., Acc. Chem. Res. 2019, 52, 1575–1587; - PubMed
-
- Bunz U. H. F., Acc. Chem. Res. 2015, 48, 1676–1686; - PubMed
-
- Bunz U. H. F., Engelhart J. U., Lindner B. D., Schaffroth M., Angew. Chem. Int. Ed. 2013, 52, 3810–3821; - PubMed
- Angew. Chem. 2013, 125, 3898–3910;
-
- Kohl B., Rominger F., Mastalerz M., Angew.Chem. 2015, 127, 6149–6154; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources