Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma
- PMID: 31609651
- PMCID: PMC6881098
- DOI: 10.1200/JCO.19.01389
Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma
Abstract
Purpose: Patients with relapsed or refractory primary mediastinal large B-cell lymphoma (rrPMBCL) have a poor prognosis, and their treatment represents an urgent and unmet need. Because PMBCL is associated with genetic aberrations at 9p24 and overexpression of programmed cell death-1 (PD-1) ligands (PD-L1), it is hypothesized to be susceptible to PD-1 blockade.
Methods: In the phase IB KEYNOTE-013 (ClinicalTrials.gov identifier: NCT01953692) and phase II KEYNOTE-170 (ClinicalTrials.gov identifier: NCT02576990) studies, adults with rrPMBCL received pembrolizumab for up to 2 years or until disease progression or unacceptable toxicity. The primary end points were safety and objective response rate in KEYNOTE-013 and objective response rate in KEYNOTE-170. Secondary end points included duration of response, progression-free survival, overall survival, and safety. Exploratory end points included association between biomarkers and pembrolizumab activity.
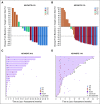
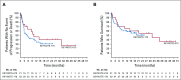
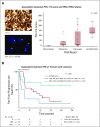
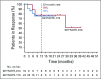
Results: The objective response rate was 48% (7 complete responses; 33%) among 21 patients in KEYNOTE-013 and 45% (7 complete responses; 13%) among 53 patients in KEYNOTE-170. After a median follow-up time of 29.1 months in KEYNOTE-013 and 12.5 months in KEYNOTE-170, the median duration of response was not reached in either study. No patient with complete response experienced progression, including 2 patients with complete response for at least 1 year off therapy. Treatment-related adverse events occurred in 24% of patients in KEYNOTE-013 and 23% of patients in KEYNOTE-170. There were no treatment-related deaths. Among 42 evaluable patients, the magnitude of the 9p24 gene abnormality was associated with PD-L1 expression, which was itself significantly associated with progression-free survival.
Conclusion: Pembrolizumab is associated with high response rate, durable activity, and a manageable safety profile in patients with rrPMBCL.
Figures

Comment in
-
Checkpoint inhibitors in primary mediastinal B-cell lymphoma: a step forward in refractory/relapsing patients?Ann Transl Med. 2020 Aug;8(16):1035. doi: 10.21037/atm.2020.04.06. Ann Transl Med. 2020. PMID: 32953835 Free PMC article. No abstract available.
References
-
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes—Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. - PubMed
-
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–459. - PubMed
-
- Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: Results of the Mabthera International Trial Group study. Ann Oncol. 2011;22:664–670. - PubMed
-
- Zinzani PL, Stefoni V, Finolezzi E, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: A retrospective study. Clin Lymphoma Myeloma. 2009;9:381–385. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

