OnabotulinumtoxinA for the treatment of major depressive disorder: a phase 2 randomized, double-blind, placebo-controlled trial in adult females
- PMID: 31609787
- PMCID: PMC6903360
- DOI: 10.1097/YIC.0000000000000290
OnabotulinumtoxinA for the treatment of major depressive disorder: a phase 2 randomized, double-blind, placebo-controlled trial in adult females
Abstract
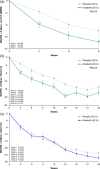
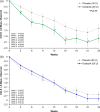
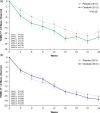
This 24-week double-blind placebo-controlled multicenter randomized phase 2 trial evaluated efficacy and safety of onabotulinumtoxinA (onabotA; BOTOX) vs. placebo for major depressive disorder (MDD) [NCT02116361]. Primary endpoint was the change in Montgomery-Åsberg Depression Rating Scale (MADRS); secondary endpoints were Clinical Global Impressions-Severity and 17-item Hamilton Depression Rating Scale at week 6. A total of 255 adult females were treated. OnabotA 30 U approached significance compared to placebo on MADRS (mixed-effect model repeated measures least-squares mean difference: -3.7; P = 0.053) and reached significance [least-squares mean differences: -3.6 to -4.2; P < 0.05 (two-sided)] at weeks 3 and 9. Secondary endpoints were also significant at several time points. At week 6, onabotA 50 U did not separate from placebo in any parameters. OnabotA was generally well-tolerated: the only treatment-emergent adverse events reported in ≥5% in either onabotA group, and more than matching placebo were headache, upper respiratory infection, and eyelid ptosis. OnabotA 30 U, administered in a standardized injection pattern in a single session, had a consistent efficacy signal across multiple depression symptom scales for 12 or more weeks. OnabotA 30 U/placebo MADRS differences of (observed ANCOVA) ≥4.0 points (up to week 15) and ≥2.0 points (weeks 18-24) agree with the 2-point change threshold considered clinically relevant in MDD. OnabotA is a local therapy and is not commonly associated with systemic effects of conventional antidepressants and may represent a novel treatment option for MDD.
Figures
References
-
- Almeida ART, Kadunc BV, Marques ERMC. Glabellar wrinkles: a pilot study of contraction patterns. Surg Cosmet Dermatol. 2010; 2:23–28
-
- American Psychiatric Association ; American Psychiatric Association Diagnostic and statistical manual of mental disorders. 20004th ed. text rev, Washington, DC: American Psychiatric Association
-
- Baldessarini RJ, Marsh E. Fluoxetine and side effects. Arch Gen Psychiatry. 1990; 47:191–192 - PubMed
-
- Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, et al. Morbidity in depressive disorders. Psychother Psychosom. 2017; 86:65–72 - PubMed
-
- BOTOX Cosmetic ; BOTOX Cosmetic (onabotulinumtoxinA) [package insert]. 2015, Irvine, CA: Allergan plc
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

