Clinical Consequences of Using an Indeterminate Range for Early Infant Diagnosis of HIV: A Decision Model
- PMID: 31609928
- PMCID: PMC7734593
- DOI: 10.1097/QAI.0000000000002155
Clinical Consequences of Using an Indeterminate Range for Early Infant Diagnosis of HIV: A Decision Model
Abstract
Background: To minimize false-positive diagnoses of HIV in exposed infants, the World Health Organization recommends confirmatory testing for all infants initiating antiretroviral therapy (ART). In settings where confirmatory testing is not feasible or intermittently performed, clinical decisions may be aided by semi-quantitative cycle thresholds (Cts) that identify positive results most likely to be false-positive.
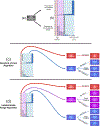
Methods: We developed a decision analysis model of HIV-exposed infants in sub-Saharan Africa to estimate the clinical consequences of deferring ART for infants with weakly positive ("indeterminate") results. We assessed the degree to which "indeterminate" results may reduce the number of infants starting ART unnecessarily while missing a small number of HIV-infected infants. Our primary outcome was the ratio of averted unnecessary ART regimens to additional HIV-related deaths (due to false-negative diagnosis) at different Ct cutoffs.
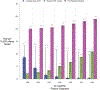
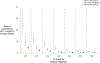
Results: The clinical consequences of adopting an indeterminate range varied with the prevalence of HIV and Ct cutoff. Considering a Ct cutoff ≥33, adopting an indeterminate range could prevent a median of 1.4 infants from receiving ART unnecessarily (95% UR: 1.0-2.0) for each additional HIV-related death. This ratio could be improved by prioritizing infants with indeterminate results for confirmatory testing [median 8.8 (95% UR: 6.0-13.3)] and by adopting a higher cutoff [median 82.3 (95% UR: 49.0-155.8) with Ct ≥36].
Conclusions: When implemented in settings where confirmatory testing is not universal, the benefits of classifying weakly positive results as "indeterminate" may outweigh the risks. Accordingly, the World Health Organization has recommended Ct values ≥33 be considered indeterminate for infant HIV diagnosis.
Figures

References
-
- Gumede-moyo S, Filteau S, Munthali T, Todd J, Musonda P. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa: a systematic literature review. Medicine (Baltimore). 2017;96(40):e8005. - PMC - PubMed
-
- Feucht U, Forsyth B, Kruger M. False-positive HIV DNA PCR testing of infants: implications in a changing epidemic. South African Med J. 2012;102(3):149–152. - PubMed
-
- World Health Organization (WHO). WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children. Geneva, Switzerland; 2010. - PubMed
-
- World Health Organization (WHO). WHO Reminds National Programmes to Retest All Newly Diagnosed People with HIV. Geneva, Switzerland; 2014.
-
- Flynn D, Johnson C, Sands A, Wong V, Baggaley R. Annex 2: An analysis of 48 national HIV testing and counselling policies. Consolidated guidelines on HIV testing services. http://apps.who.int/iris/bitstream/10665/180208/1/WHO_HIV_2015.19_eng.pdf. Published 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

