Current Practices and Novel Techniques in the Diagnosis and Management of Neuroendocrine Tumors of Unknown Primary
- PMID: 31609931
- PMCID: PMC6830950
- DOI: 10.1097/MPA.0000000000001391
Current Practices and Novel Techniques in the Diagnosis and Management of Neuroendocrine Tumors of Unknown Primary
Abstract
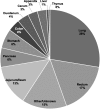
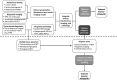
Neuroendocrine tumors (NETs) comprise a heterogeneous group of neoplasms in which tumor staging/prognosis and response to treatments depend heavily on accurate and timely identification of the anatomic primary site or NET subtype. Despite recent technological advancements and use of multiple diagnostic modalities, 10% to 14% of newly diagnosed NETs are not fully characterized based on subtype or anatomic primary site. Inability to fully characterize NETs of unknown primary may cause delays in surgical intervention and limit potential treatment options. To address this unmet need, clinical validity and utility are being demonstrated for novel approaches that improve NET subtype or anatomic primary site identification. Functional imaging using Ga-radiolabeled DOTATATE positron emission tomography/computed tomography has been shown to overcome some false-positive and resolution issues associated with octreotide scanning and computed tomography/magnetic resonance imaging. Using a genomic approach, molecular tumor classification based on differential gene expression has demonstrated high diagnostic accuracy in blinded validation studies of different NET types and subtypes. Given the widespread availability of these technologies, we propose an algorithm for the workup of NETs of unknown primary that integrates these approaches. Including these technologies in the standard workup will lead to better NET subtype identification and improved treatment optimization for patients.
Conflict of interest statement
A.E.H. has received consulting fees from Novartis and Ipsen Biopharmaceuticals Inc. R.A.R. serves as a consultant for Ipsen Biopharmaceuticals Inc and Biotheranostics Inc, as well as a speaker for Merck & Co Inc, Genentech, Astra Zeneca, and Ipsen Biopharmaceuticals. E.L. has received personal fees from Wren Laboratories, been part of Speaker's Bureau for Novartis, been a consultant for Ipsen Biopharmaceuticals Inc, received consulting fees from Novartis and Ipsen, and been a speaker for Lexicon and Advanced Accelerator Applications. L.B.A. declares no conflicts of interest.
Figures
References
-
- Zandee WT, Kamp K, van Adrichem RC, et al. Effect of hormone secretory syndromes on neuroendocrine tumor prognosis. Endocr Relat Cancer. 2017;24:R261–R274. - PubMed
-
- Hauso O, Gustafsson BI, Kidd M, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–2664. - PubMed
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. - PubMed
-
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. - PubMed

