An innovative non-invasive technique for subcutaneous tumour measurements
- PMID: 31609977
- PMCID: PMC6791540
- DOI: 10.1371/journal.pone.0216690
An innovative non-invasive technique for subcutaneous tumour measurements
Abstract
Introduction: In oncological drug development, animal studies continue to play a central role in which the volume of subcutaneous tumours is monitored to assess the efficacy of new drugs. The tumour volume is estimated by taking the volume to be that of a regular spheroid with the same dimensions. However, this method is subjective, insufficiently traceable, and is subject to error in the accuracy of volume estimates as tumours are frequently irregular.
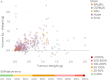
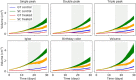
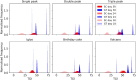
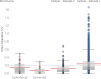
Methods & results: This paper reviews the standard technique for tumour volume assessment, calliper measurements, by conducting a statistical review of a large dataset consisting of 2,500 tumour volume measurements from 1,600 mice by multiple operators across 6 mouse strains and 20 tumour models. Additionally, we explore the impact of six different tumour morphologies on volume estimation and the detection of treatment effects using a computational tumour growth model. Finally, we propose an alternative method to callipers for estimating volume-BioVolumeTM, a 3D scanning technique. BioVolume simultaneously captures both stereo RGB (Red, Green and Blue) images from different light sources and infrared thermal images of the tumour in under a second. It then detects the tumour region automatically and estimates the tumour volume in under a minute. Furthermore, images can be processed in parallel within the cloud and so the time required to process multiple images is similar to that required for a single image. We present data of a pre-production unit test consisting of 297 scans from over 120 mice collected by four different operators.
Conclusion: This work demonstrates that it is possible to record tumour measurements in a rapid minimally invasive, morphology-independent way, and with less human-bias compared to callipers, whilst also improving data traceability. Furthermore, the images collected by BioVolume may be useful, for example, as a source of biomarkers for animal welfare and secondary drug toxicity / efficacy.
Conflict of interest statement
Fuel3D is developing BioVolume and claims financial competing interests on the product. The data presented here is generated as a pre-production test on the technology. There are not specific patents granted or filed for this technology or any part of it. AstraZeneca and Medimmune do not claim any financial interests. This does not alter adherence to PLOS policies on sharing data.
Figures





References
-
- Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989; - PubMed
-
- Euhus DM, Hudd C, Laregina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986; - PubMed
-
- Ishimori T, Tatsumi M, Wahl RL. Tumor response assessment is more robust with sequential CT scanning than external caliper measurements. Acad Radiol. 2005; - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

