Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons
- PMID: 31611014
- PMCID: PMC6889021
- DOI: 10.1016/j.brs.2019.10.002
Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons
Abstract
Background: Transcranial magnetic stimulation (TMS) enables non-invasive modulation of brain activity with both clinical and research applications, but fundamental questions remain about the neural types and elements TMS activates and how stimulation parameters affect the neural response.
Objective: To develop a multi-scale computational model to quantify the effect of TMS parameters on the direct response of individual neurons.
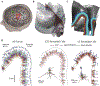
Methods: We integrated morphologically-realistic neuronal models with TMS-induced electric fields computed in a finite element model of a human head to quantify the cortical response to TMS with several combinations of pulse waveforms and current directions.
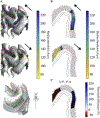
Results: TMS activated with lowest intensity intracortical axonal terminations in the superficial gyral crown and lip regions. Layer 5 pyramidal cells had the lowest thresholds, but layer 2/3 pyramidal cells and inhibitory basket cells were also activated at most intensities. Direct activation of layers 1 and 6 was unlikely. Neural activation was largely driven by the field magnitude, rather than the field component normal to the cortical surface. Varying the induced current direction caused a waveform-dependent shift in the activation site and provided a potential mechanism for experimentally observed differences in thresholds and latencies of muscle responses.
Conclusions: This biophysically-based simulation provides a novel method to elucidate mechanisms and inform parameter selection of TMS and other cortical stimulation modalities. It also serves as a foundation for more detailed network models of the response to TMS, which may include endogenous activity, synaptic connectivity, inputs from intrinsic and extrinsic axonal projections, and corticofugal axons in white matter.
Keywords: Finite element method; Motor cortex; Neuron models; Simulation; Transcranial magnetic stimulation.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
B.W. is inventor on patent applications on technology for TMS. A.V.P. is inventor on patents and patent applications on TMS technology; in the past 3 years, he has received travel support as well as patent royalties from Rogue Research for cTMS; research and travel support, consulting fees, as well as equipment donations from Tal Medical/Neurex Therapeutics; patent application and research support as well as hardware donations from Magstim; as well as equipment loans and hardware donations from MagVenture, all related to TMS.
Figures






Comment in
-
Does TMS of the precentral motor hand knob primarily stimulate the dorsal premotor cortex or the primary motor hand area?Brain Stimul. 2020 Mar-Apr;13(2):517-518. doi: 10.1016/j.brs.2019.12.015. Epub 2019 Dec 17. Brain Stimul. 2020. PMID: 31924573 No abstract available.
References
-
- Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–206. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

