Biology and therapeutic potential of interleukin-10
- PMID: 31611251
- PMCID: PMC7037253
- DOI: 10.1084/jem.20190418
Biology and therapeutic potential of interleukin-10
Abstract
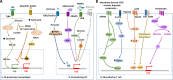
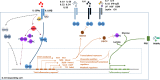
The cytokine IL-10 is a key anti-inflammatory mediator ensuring protection of a host from over-exuberant responses to pathogens and microbiota, while playing important roles in other settings as sterile wound healing, autoimmunity, cancer, and homeostasis. Here we discuss our current understanding of the regulation of IL-10 production and of the molecular pathways associated with IL-10 responses. In addition to IL-10's classic inhibitory effects on myeloid cells, we also describe the nonclassic roles attributed to this pleiotropic cytokine, including how IL-10 regulates basic processes of neural and adipose cells and how it promotes CD8 T cell activation, as well as epithelial repair. We further discuss its therapeutic potential in the context of different diseases and the outstanding questions that may help develop an effective application of IL-10 in diverse clinical settings.
© 2019 Saraiva et al.
Figures


References
-
- Amiel E., Everts B., Freitas T.C., King I.L., Curtis J.D., Pearce E.L., and Pearce E.J.. 2012. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J. Immunol. 189:2151–2158. 10.4049/jimmunol.1103741 - DOI - PMC - PubMed
-
- An H., Xu H., Zhang M., Zhou J., Feng T., Qian C., Qi R., and Cao X.. 2005. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism. Blood. 105:4685–4692. 10.1182/blood-2005-01-0191 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

