Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities
- PMID: 31611368
- PMCID: PMC6825284
- DOI: 10.1073/pnas.1908079116
Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities
Abstract
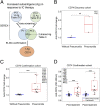
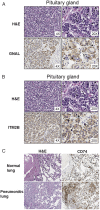
Immune checkpoint (IC) therapy provides substantial benefits to cancer patients but can also cause distinctive toxicities termed immune-related adverse events (irAEs). Biomarkers to predict toxicities will be necessary to improve management of patients receiving IC therapy. We relied on serological analysis of recombinant cDNA expression libraries to evaluate plasma samples from patients treated with IC therapy and identified autoantibodies, both in pretreatment and on-treatment samples prior to the development of irAEs, which correlate with the development of immune-related hypophysitis (anti-GNAL and anti-ITM2B autoantibodies) and pneumonitis (anti-CD74 autoantibody). We developed an enzyme-linked immunosorbent assay and tested additional patient samples to confirm our initial findings. Collectively, our data suggest that autoantibodies may correlate with irAEs related to IC therapy, and specific autoantibodies may be detected early for the management of irAEs.
Keywords: autoimmune antibody; hypophysitis; immune checkpoint therapy; immune-related adverse events; pneumonitis.
Conflict of interest statement
Competing interest statement: J.P.A. has ownership in Jounce, Neon, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Hummingbird, Dragonfly, Lytix, and Tvardi Therapeutics and serves as a consultant for Jounce, Kite Pharma, Neon, Amgen, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Tvardi Therapeutics, Lytix, Hummingbird, and Dragonfly. P.S. has ownership in Jounce, Neon, Constellation, Oncolytics, BioAtla, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAb, Lytix, Hummingbird, and Dragonfly and serves as a consultant for Constellation, Jounce, Kite Pharma, Neon, BioAtla, Pieris Pharmaceuticals, Oncolytics Biotech, Merck, BioMx, Forty-Seven, Polaris, Apricity, Marker Therapeutics, Codiak, ImaginAb, Hummingbird, Lytix, and Dragonfly. J.G. serves as a consultant for ARMO Biosciences, AstraZeneca, CRISPR Therapeutics, Jounce, Nektar, Polaris, Pfizer, and Symphogen. C.G.D. and J.P.A. are coauthors on a 2016 workshop report.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

