Differential development of large-cell neuroendocrine or small-cell lung carcinoma upon inactivation of 4 tumor suppressor genes
- PMID: 31611390
- PMCID: PMC6825275
- DOI: 10.1073/pnas.1821745116
Differential development of large-cell neuroendocrine or small-cell lung carcinoma upon inactivation of 4 tumor suppressor genes
Abstract
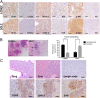
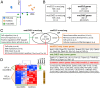
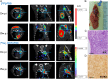
High-grade neuroendocrine lung malignancies (large-cell neuroendocrine cell carcinoma, LCNEC, and small-cell lung carcinoma, SCLC) are among the most deadly lung cancer conditions with no optimal clinical management. The biological relationships between SCLC and LCNEC are still largely unknown and a current matter of debate as growing molecular data reveal high heterogeneity with potential therapeutic consequences. Here we describe murine models of high-grade neuroendocrine lung carcinomas generated by the loss of 4 tumor suppressors. In an Rbl1-null background, deletion of Rb1, Pten, and Trp53 floxed alleles after Ad-CMVcre infection in a wide variety of lung epithelial cells produces LCNEC. Meanwhile, inactivation of these genes using Ad-K5cre in basal cells leads to the development of SCLC, thus differentially influencing the lung cancer type developed. So far, a defined model of LCNEC has not been reported. Molecular and transcriptomic analyses of both models revealed strong similarities to their human counterparts. In addition, a 68Ga-DOTATOC-based molecular-imaging method provides a tool for detection and monitoring the progression of the cancer. These data offer insight into the biology of SCLC and LCNEC, providing a useful framework for development of compounds and preclinical investigations in accurate immunocompetent models.
Keywords: LCNEC; SCLC; cell of origin; tumor suppressor.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Travis W. D., et al. ; WHO Panel , The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 10, 1243–1260 (2015). - PubMed
-
- Miyoshi T., et al. , Genomic profiling of large-cell neuroendocrine carcinoma of the lung. Clin. Cancer Res. 23, 757–765 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

