Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort
- PMID: 31611612
- PMCID: PMC6888839
- DOI: 10.1038/s41416-019-0587-2
Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort
Erratum in
-
Correction to: Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort.Br J Cancer. 2022 Mar;126(5):831. doi: 10.1038/s41416-021-01678-2. Br J Cancer. 2022. PMID: 34949789 Free PMC article. No abstract available.
Abstract
Background: Guidelines remain unclear over whether patients with early stage oral cancer without overt neck disease benefit from upfront elective neck dissection (END), particularly those with the smallest tumours.
Methods: We conducted a randomised trial of patients with stage T1/T2 N0 disease, who had their mouth tumour resected either with or without END. Data were also collected from a concurrent cohort of patients who had their preferred surgery. Endpoints included overall survival (OS) and disease-free survival (DFS). We conducted a meta-analysis of all six randomised trials.
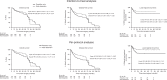
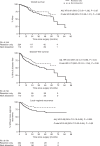
Results: Two hundred fifty randomised and 346 observational cohort patients were studied (27 hospitals). Occult neck disease was found in 19.1% (T1) and 34.7% (T2) patients respectively. Five-year intention-to-treat hazard ratios (HR) were: OS HR = 0.71 (p = 0.18), and DFS HR = 0.66 (p = 0.04). Corresponding per-protocol results were: OS HR = 0.59 (p = 0.054), and DFS HR = 0.56 (p = 0.007). END was effective for small tumours. END patients experienced more facial/neck nerve damage; QoL was largely unaffected. The observational cohort supported the randomised findings. The meta-analysis produced HR OS 0.64 and DFS 0.54 (p < 0.001).
Conclusion: SEND and the cumulative evidence show that within a generalisable setting oral cancer patients who have an upfront END have a lower risk of death/recurrence, even with small tumours.
Clinical trial registration: NIHR UK Clinical Research Network database ID number: UKCRN 2069 (registered on 17/02/2006), ISCRTN number: 65018995, ClinicalTrials.gov Identifier: NCT00571883.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Comment on "Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort".Br J Cancer. 2020 Sep;123(7):1202-1203. doi: 10.1038/s41416-020-0982-8. Epub 2020 Jul 16. Br J Cancer. 2020. PMID: 32669673 Free PMC article. No abstract available.
-
Comment on "Nationwide randomised trial evaluating elective neck dissection for early-stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort.".Br J Cancer. 2020 Sep;123(7):1198-1199. doi: 10.1038/s41416-020-0981-9. Epub 2020 Jul 16. Br J Cancer. 2020. PMID: 32669674 Free PMC article. No abstract available.
-
Reply to Comment(s) on "Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort".Br J Cancer. 2020 Sep;123(7):1200-1201. doi: 10.1038/s41416-020-0983-7. Epub 2020 Jul 16. Br J Cancer. 2020. PMID: 32669675 Free PMC article. No abstract available.
References
-
- Ghantous Y, Abu Elnaaj I. Global incidence and risk factors of oral cancer. Harefuah. 2017;156:645–649. - PubMed
-
- Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (2019).
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

