Differential effects of luteolin and its glycosides on invasion and apoptosis in MDA-MB-231 triple-negative breast cancer cells
- PMID: 31611756
- PMCID: PMC6785773
- DOI: 10.17179/excli2019-1459
Differential effects of luteolin and its glycosides on invasion and apoptosis in MDA-MB-231 triple-negative breast cancer cells
Abstract
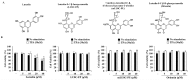
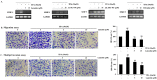
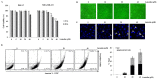
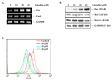
Luteolin is known to have anticancer activity in various cancers. Recent studies have shown that luteolin glycosides, such as luteolin-8-C-β-fucopyranoside, 7-methoxy-luteolin-8-C-β-(6- deoxyxylopyranos-3-uloside) and luteolin-8-C-β-d-glucopyranoside, flavonoids that are present in Arthraxon hispidus, exert antimigratory and anti-invasive effects, but no cytotoxic effect in estrogen receptor-positive MCF7 breast cancer cells. In the present study, we further investigated and compared differential effects of luteolin and its glycosides in MDA-MB-231 triple-negative breast cancer cells. Luteolin suppressed the expression of matrix metalloproteinase-9 and inhibited migration and invasion in MDA-MB-231 cells treated with the tumor promotor 12-O-tetradecanoylphorbol-13-acetate at non-cytotoxic concentrations (0, 5, and 10 μM). Furthermore, at cytotoxic concentrations (20 and 40 μM), luteolin induced apoptosis via extrinsic and intrinsic pathways in MDA-MB-231 cells. However, luteolin glycosides did not exert any cytotoxic, antimigratory, or anti-invasive effect in MDA-MB-231 cells. In brief, luteolin had both antimetastatic and cytotoxic effects on MDA-MB-231 cells, whereas luteolin glycosides had no effect on this cell line. Taking together the present results and our previous findings on the differential effects of luteolin and its glycosides on MDA-MB-231 and MCF-7 breast cancer cells, luteolin and its glycosides can be suggested as a potential candidate for breast cancer therapy.
Keywords: apoptosis; breast cancer; invasion; luteolin; tumor migration.
Copyright © 2019 Lee et al.
Figures

References
-
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. - PubMed
-
- Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–358. - PubMed
-
- Bak Y, Ham S, Baatartsogt O, Jung SH, Choi KD, Han TY, et al. A1E inhibits proliferation and induces apoptosis in NCI-H460 lung cancer cells via extrinsic and intrinsic pathways. Mol Biol Rep. 2013;40:4507–4519. - PubMed
-
- Choi J, Gyamfi J, Jang H, Koo JS. The role of tumor-associated macrophage in breast cancer biology. Histol Histopathol. 2018;33:133–145. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
