Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis
- PMID: 31611899
- PMCID: PMC6776609
- DOI: 10.3389/fpls.2019.01186
Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis
Abstract
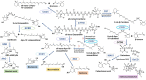
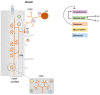
Plants utilize hormones and other small molecules to trigger and coordinate their growth and developmental processes, adapt and respond to environmental cues, and communicate with surrounding organisms. Some of these molecules originate from carotenoids that act as universal precursors of bioactive metabolites arising through oxidation of the carotenoid backbone. This metabolic conversion produces a large set of compounds known as apocarotenoids, which includes the plant hormones abscisic acid (ABA) and strigolactones (SLs) and different signaling molecules. An increasing body of evidence suggests a crucial role of previously identified and recently discovered carotenoid-derived metabolites in the communication with arbuscular mycorrhizal (AM) fungi and the establishment of the corresponding symbiosis, which is one of the most relevant plant-fungus mutualistic interactions in nature. In this review, we provide an update on the function of apocarotenoid hormones and regulatory metabolites in AM symbiosis, highlighting their effect on both partners.
Keywords: abscisic acid; apocarotenoids; arbuscular mycorrhizal symbiosis; blumenols; carotenoids; mycorradicin; strigolactones; zaxinone.
Copyright © 2019 Fiorilli, Wang, Bonfante, Lanfranco and Al-Babili.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

