Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy
- PMID: 31612267
- PMCID: PMC6866660
- DOI: 10.1007/s00125-019-04998-4
Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy
Abstract
Aims/hypothesis: Levels of neutrophil elastase, a serine protease secreted by neutrophils, are elevated in diabetes. The purpose of this study was to determine whether neutrophil elastase (NE) contributes to the diabetes-induced increase in retinal vascular permeability in mice with streptozotocin-induced diabetes, and, if so, to investigate the potential role of IL-17 in this process.
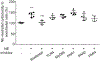
Methods: In vivo, diabetes was induced in neutrophil elastase-deficient (Elane-/-), Il-17a-/- and wild-type mice. After 8 months of diabetes, Elane-/- mice and wild-type age-matched control mice were injected with FITC-BSA. Fluorescence microscopy was used to assess leakage of FITC-BSA from the retinal vasculature into the neural retina. The level of NE in Il-17a-/- diabetic retina and sera were determined by ELISA. In vitro, the effect of NE on the permeability and viability of human retinal endothelial cells and the expression of junction proteins and adhesion molecules were studied.
Results: Eight months of diabetes resulted in increased retinal vascular permeability and levels of NE in retina and plasma of wild-type animals. All of these abnormalities were significantly inhibited in mice lacking the elastase. The diabetes-induced increase in NE was inhibited in mice lacking IL-17. In vitro, NE increased retinal endothelial cell permeability, which was partially inhibited by a myeloid differentiation primary response 88 (MyD88) inhibitor, NF-κB inhibitor, and protease-activated receptor (PAR)2 inhibitor. NE degraded vascular endothelial-cadherin (VE-cadherin) in a concentration-dependent manner.
Conclusions/interpretation: IL-17 regulates NE expression in diabetes. NE contributes to vascular leakage in diabetic retinopathy, partially through activation of MyD88, NF-κB and PAR2 and degradation of VE-cadherin.
Keywords: Diabetic retinopathy; Elane; IL-17; Neutrophil elastase; Vascular permeability.
Conflict of interest statement
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Figures





References
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. (2017) IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128: 40–50 - PubMed
-
- Engelgau MM, Geiss LS, Saaddine JB, et al. (2004) The evolving diabetes burden in the United States. Annals of internal medicine 140: 945–950 - PubMed
-
- Klaassen I, Van Noorden CJ, Schlingemann RO (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Progress in retinal and eye research 34: 19–48 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

