Probing force in living cells with optical tweezers: from single-molecule mechanics to cell mechanotransduction
- PMID: 31612379
- PMCID: PMC6815294
- DOI: 10.1007/s12551-019-00599-y
Probing force in living cells with optical tweezers: from single-molecule mechanics to cell mechanotransduction
Abstract
The invention of optical tweezers more than three decades ago has opened new avenues in the study of the mechanical properties of biological molecules and cells. Quantitative force measurements still represent a challenging task in living cells due to the complexity of the cellular environment. Here, we review different methodologies to quantitatively measure the mechanical properties of living cells, the strength of adhesion/receptor bonds, and the active force produced during intracellular transport, cell adhesion, and migration. We discuss experimental strategies to attain proper calibration of optical tweezers and molecular resolution in living cells. Finally, we show recent studies on the transduction of mechanical stimuli into biomolecular and genetic signals that play a critical role in cell health and disease.
Keywords: adhesion; cell mechanics; force spectroscopy; mechanotransduction; molecular motors; optical tweezers.
Figures
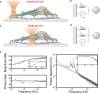
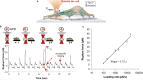




References
-
- Almonacid M, Ahmed WW, Bussonnier M, Mailly P, Betz T, Voituriez R, Gov NS, Verlhac M-H. Active diffusion positions the nucleus in mouse oocytes. Nat Cell Biol. 2015;17(4):470–479. - PubMed
-
- Arya, Maneesh, Bahman Anvari, Gabriel M. Romo, Miguel A. Cruz, Jing-Fei Dong, Larry V McIntire, Joel L. Moake, and José A. Ló pez (2002) “Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers” - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

