Behavioral Pharmacology of Novel Kappa Opioid Receptor Antagonists in Rats
- PMID: 31613314
- PMCID: PMC7145521
- DOI: 10.1093/ijnp/pyz054
Behavioral Pharmacology of Novel Kappa Opioid Receptor Antagonists in Rats
Abstract
Background: New treatments for stress-related disorders including depression, anxiety, and substance use disorder are greatly needed. Kappa opioid receptors are expressed in the central nervous system, including areas implicated in analgesia and affective state. Although kappa opioid receptor agonists share the antinociceptive effects of mu opioid receptor agonists, they also tend to produce negative affective states. In contrast, selective kappa opioid receptor antagonists have antidepressant- and anxiolytic-like effects, stimulating interest in their therapeutic potential. The prototypical kappa opioid receptor antagonists (e.g., norBNI, JDTic) have an exceptionally long duration of action that complicates their use in humans, particularly in tests to establish safety. This study was designed to test dose- and time-course effects of novel kappa opioid receptor antagonists with the goal of identifying short-acting lead compounds for future medication development.
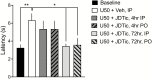
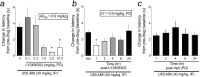
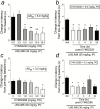
Methods: We screened 2 novel, highly selective kappa opioid receptor antagonists (CYM-52220 and CYM-52288) with oral efficacy in the warm water tail flick assay in rats to determine initial dose and time course effects. For comparison, we tested existing kappa opioid receptor antagonists JDTic and LY-2456302 (also known as CERC-501 or JNJ-67953964).
Results: In the tail flick assay, the rank order of duration of action for the antagonists was LY-2456302 < CYM-52288 < CYM-52220 << JDTic. Furthermore, LY-2456302 blocked the depressive (anhedonia-producing) effects of the kappa opioid receptor agonist U50,488 in the intracranial self-stimulation paradigm, albeit at a higher dose than that needed for analgesic blockade in the tail flick assay.
Conclusions: These results suggest that structurally diverse kappa opioid receptor antagonists can have short-acting effects and that LY-2456302 reduces anhedonia as measured in the intracranial self-stimulation test.
Keywords: JDTic; 488; ICSS; LY-2456302; U50; analgesia.
© The Author(s) 2019. Published by Oxford University Press on behalf of CINP.
Figures

References
-
- Barrett AC, Cook CD, Terner JM, Roach EL, Syvanthong C, Picker MJ (2002) Sex and rat strain determine sensitivity to kappa opioid-induced antinociception. Psychopharmacology (Berl) 160:170–181. - PubMed
-
- Beardsley PM, Howard JL, Shelton KL, Carroll FI (2005) Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 183:118–126. - PubMed
-
- BlackThorn (2018) Blackthorn therapeutics initiates phase 1 study of BTRX-335140, an Investigational Kappa Opioid Receptor (KOR) antagonist. In: BlackThorn Therapeutics; San Francisco, CA. https://www.blackthornrx.com/btrx-335140-kappa-opioid-receptor-antagonist/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

