Phosphorus-mediated alleviation of aluminum toxicity revealed by the iTRAQ technique in Citrus grandis roots
- PMID: 31613915
- PMCID: PMC6793874
- DOI: 10.1371/journal.pone.0223516
Phosphorus-mediated alleviation of aluminum toxicity revealed by the iTRAQ technique in Citrus grandis roots
Abstract
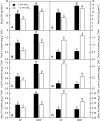
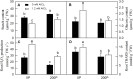
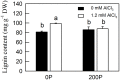
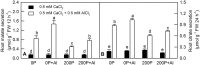
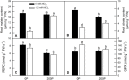
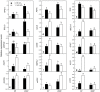
Citrus grandis seedlings were irrigated with nutrient solutions with four Al-P combinations [two Al levels (0 mM and 1.2 mM AlCl3·6H2O) × two P levels (0 μM and 200 μM KH2PO4)] for 18 weeks. Al dramatically inhibited the growth of C. grandis seedlings, as revealed by a decreased dry weight of roots and shoots. Elevating P level could ameliorate the Al-induced growth inhibition and organic acid (malate and citrate) secretion in C. grandis. Using a comparative proteomic approach revealed by the isobaric tags for relative and absolute quantification (iTRAQ) technique, 318 differentially abundant proteins (DAPs) were successfully identified and quantified in this study. The possible mechanisms underlying P-induced alleviation of Al toxicity in C. grandis were proposed. Furthermore, some DAPs, such as GLN phosphoribosyl pyrophosphate amidotransferase 2, ATP-dependent caseinolytic (Clp) protease/crotonase family protein, methionine-S-oxide reductase B2, ABC transporter I family member 17 and pyridoxal phosphate phosphatase, were reported for the first time to respond to Al stress in Citrus plants. Our study provides some proteomic details about the alleviative effects of P on Al toxicity in C. grandis, however, the exact function of the DAPs identified herein in response to Al tolerance in plants must be further investigated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Kumari M, Taylor GJ, Deyholos MK: Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Molecular Genetics & Genomics 2008; 279(4):339–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

