Exosomes and STUB1/CHIP cooperate to maintain intracellular proteostasis
- PMID: 31613922
- PMCID: PMC6794069
- DOI: 10.1371/journal.pone.0223790
Exosomes and STUB1/CHIP cooperate to maintain intracellular proteostasis
Abstract
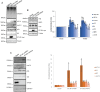
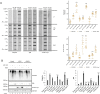
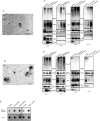
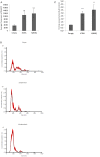
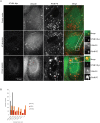
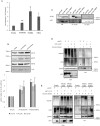
Deregulation of proteostasis is a main feature of many age-related diseases, often leading to the accumulation of toxic oligomers and insoluble protein aggregates that accumulate intracellularly or in the extracellular space. To understand the mechanisms whereby toxic or otherwise unwanted proteins are secreted to the extracellular space, we inactivated the quality-control and proteostasis regulator ubiquitin ligase STUB1/CHIP. Data indicated that STUB1 deficiency leads both to the intracellular accumulation of protein aggregates and to an increase in the secretion of small extracellular vesicles (sEVs), including exosomes. Secreted sEVs are enriched in ubiquitinated and/or undegraded proteins and protein oligomers. Data also indicates that oxidative stress induces an increase in the release of sEVs in cells depleted from STUB1. Overall, the results presented here suggest that cells use exosomes to dispose of damaged and/or undegraded proteins as a means to reduce intracellular accumulation of proteotoxic material.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

