A customizable microfluidic platform for medium-throughput modeling of neuromuscular circuits
- PMID: 31614290
- PMCID: PMC7294901
- DOI: 10.1016/j.biomaterials.2019.119537
A customizable microfluidic platform for medium-throughput modeling of neuromuscular circuits
Abstract
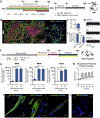
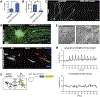
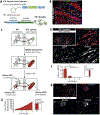
Neuromuscular circuits (NMCs) are vital for voluntary movement, and effective models of NMCs are needed to understand the pathogenesis of, as well as to identify effective treatments for, multiple diseases, including Duchenne's muscular dystrophy and amyotrophic lateral sclerosis. Microfluidics are ideal for recapitulating the central and peripheral compartments of NMCs, but myotubes often detach before functional NMCs are formed. In addition, microfluidic systems are often limited to a single experimental unit, which significantly limits their application in disease modeling and drug discovery. Here, we developed a microfluidic platform (MFP) containing over 100 experimental units, making it suitable for medium-throughput applications. To overcome detachment, we incorporated a reactive polymer surface allowing customization of the environment to culture different cell types. Using this approach, we identified conditions that enable long-term co-culture of human motor neurons and myotubes differentiated from human induced pluripotent stem cells inside our MFP. Optogenetics demonstrated the formation of functional NMCs. Furthermore, we developed a novel application of the rabies tracing assay to efficiently identify NMCs in our MFP. Therefore, our MFP enables large-scale generation and quantification of functional NMCs for disease modeling and pharmacological drug targeting.
Keywords: Microfluidics; Motor unit; Neuromuscular circuit; Rabies viral tracing; Skeletal muscle; poly(ethylene-alt-maleic anhydride).
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors have no competing interests to declare.
Figures




References
-
- Parone PA, Da Cruz S, Han JS, McAlonis-Downes M, Vetto AP, Lee SK, Tseng E, Cleveland DW, Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis, J. Neurosci 33 (11) (2013) 4657–4671. - PMC - PubMed
-
- Kanning KC, Kaplan A, Henderson CE, Motor neuron diversity in development and disease, Annu. Rev. Neurosci 33 (2010) 409–440. - PubMed
-
- Edens BM, Ajroud-Driss S, Ma L, Ma YC, Molecular mechanisms and animal models of spinal muscular atrophy, Biochim. Biophys. Acta 1852 (4) (2015) 685–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

