Scaffold-Free Endometrial Organoids Respond to Excess Androgens Associated With Polycystic Ovarian Syndrome
- PMID: 31614364
- PMCID: PMC7112974
- DOI: 10.1210/clinem/dgz100
Scaffold-Free Endometrial Organoids Respond to Excess Androgens Associated With Polycystic Ovarian Syndrome
Abstract
Context: Polycystic ovary syndrome (PCOS) is a prevalent disorder in reproductive aged women associated with a number of endocrine and metabolic complications, including increased risk of endometrial cancer.
Objective: To study the effect of the characteristic increased androgen levels in PCOS on the endometrium, a novel scaffold-free multicellular endometrial organoid was established.
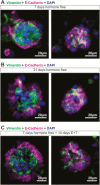
Design: Human endometrial organoids were constructed using primary endometrial epithelial and stromal cells from endometrial tissues. Organoids were treated for 14 days with physiologic levels of estradiol and testosterone to mimic a normal follicular phase or PCOS hormone profiles. Organoids were harvested for immunostaining and ribonucleic acid sequencing.
Setting: Academic institution.
Patients: Endometrial tissues from 10 premenopausal women undergoing hysterectomy for benign pathologies were obtained following written consent.
Main outcome measures: Organoid architecture, cell specific markers, functional markers, proliferation, and gene expression were measured.
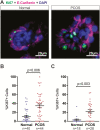
Results: A method to generate scaffold-free endometrial organoids containing epithelial and stromal cells was established. These organoids exhibited distinct organization with epithelial cells lining the outer surface and stromal cells in the center of the organoids. Epithelial cells were polarized, organoids expressed cell type specific and functional markers, as well as androgen, estrogen, and progesterone receptors. Treatment with PCOS hormones increased cell proliferation and dysregulated genes in endometrial organoids.
Conclusions: A new multicellular, scaffold-free endometrial organoid system was established that resembled physiology of the native endometrium. Excess androgens in PCOS promoted cell proliferation in endometrial organoids, revealing new mechanisms of PCOS-associated with risk of endometrial neoplasia.
Keywords: PCOS; endometrium; organoids; polycystic ovarian syndrome.
© Endocrine Society 2019. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. - PubMed
-
- Piltonen TT. Polycystic ovary syndrome: endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2016;37:66–79. - PubMed
-
- Stracquadanio M, Ciotta L, Palumbo MA. Relationship between serum anti-Mullerian hormone and intrafollicular AMH levels in PCOS women. Gynecol Endocrinol. 2018;34(3):223–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

