New Imaging Markers of Clinical Outcome in Asymptomatic Patients with Severe Aortic Regurgitation
- PMID: 31614523
- PMCID: PMC6832544
- DOI: 10.3390/jcm8101654
New Imaging Markers of Clinical Outcome in Asymptomatic Patients with Severe Aortic Regurgitation
Abstract
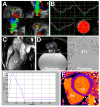
Background: Determining the value of new imaging markers to predict aortic valve (AV) surgery in asymptomatic patients with severe aortic regurgitation (AR) in a prospective, observational, multicenter study. Methods: Consecutive patients with chronic severe AR were enrolled between 2015-2018. Baseline examination included echocardiography (ECHO) with 2- and 3-dimensional (2D and 3D) vena contracta area (VCA), and magnetic resonance imaging (MRI) with regurgitant volume (RV) and fraction (RF) analyzed in CoreLab. Results: The mean follow-up was 587 days (interquartile range (IQR) 296-901) in a total of 104 patients. Twenty patients underwent AV surgery. Baseline clinical and laboratory data did not differ between surgically and medically treated patients. Surgically treated patients had larger left ventricular (LV) dimension, end-diastolic volume (all p < 0.05), and the LV ejection fraction was similar. The surgical group showed higher prevalence of severe AR (70% vs. 40%, p = 0.02). Out of all imaging markers 3D VCA, MRI-derived RV and RF were identified as the strongest independent predictors of AV surgery (all p < 0.001). Conclusions: Parameters related to LV morphology and function showed moderate accuracy to identify patients in need of early AV surgery at the early stage of the disease. 3D ECHO-derived VCA and MRI-derived RV and RF showed high accuracy and excellent sensitivity to identify patients in need of early surgery.
Keywords: T1 mapping; aortic regurgitation; echocardiography; longitudinal strain; magnetic resonance imaging; vena contracta area.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Baumgartner H., Falk V., Bax J.J., Bonis M., Hamm C., Holm P.J., Iung B., Lancelloti P., Lansac E., Rodriguez Munoz D., et al. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. - DOI - PubMed
-
- Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. - DOI - PubMed
-
- Iung B., Baron G., Butchart E.G., Delahaye F., Gohlke-Barwolf C., Levang O.W., Tornos P., Vanoverschelde J.L., Vermeer F., Boersma E., et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003;24:1231–1243. doi: 10.1016/S0195-668X(03)00201-X. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

