Polarization Induced Electro-Functionalization of Pore Walls: A Contactless Technology
- PMID: 31614545
- PMCID: PMC6956341
- DOI: 10.3390/bios9040121
Polarization Induced Electro-Functionalization of Pore Walls: A Contactless Technology
Abstract
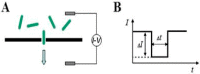
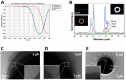
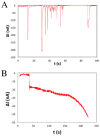
This review summarizes recent advances in micro- and nanopore technologies with a focus on the functionalization of pores using a promising method named contactless electro-functionalization (CLEF). CLEF enables the localized grafting of electroactive entities onto the inner wall of a micro- or nano-sized pore in a solid-state silicon/silicon oxide membrane. A voltage or electrical current applied across the pore induces the surface functionalization by electroactive entities exclusively on the inside pore wall, which is a significant improvement over existing methods. CLEF's mechanism is based on the polarization of a sandwich-like silicon/silicon oxide membrane, creating electronic pathways between the core silicon and the electrolyte. Correlation between numerical simulations and experiments have validated this hypothesis. CLEF-induced micro- and nanopores functionalized with antibodies or oligonucleotides were successfully used for the detection and identification of cells and are promising sensitive biosensors. This technology could soon be successfully applied to planar configurations of pores, such as restrictions in microfluidic channels.
Keywords: CLEF; biosensing; contactless; electro-functionalization; micropore; nanopore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

