Metabolism, Transport and Drug-Drug Interactions of Silymarin
- PMID: 31615114
- PMCID: PMC6832356
- DOI: 10.3390/molecules24203693
Metabolism, Transport and Drug-Drug Interactions of Silymarin
Abstract
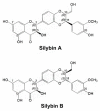
Silymarin, the extract of milk thistle, and its major active flavonolignan silybin, are common products widely used in the phytotherapy of liver diseases. They also have promising effects in protecting the pancreas, kidney, myocardium, and the central nervous system. However, inconsistent results are noted in the different clinical studies due to the low bioavailability of silymarin. Extensive studies were conducted to explore the metabolism and transport of silymarin/silybin as well as the impact of its consumption on the pharmacokinetics of other clinical drugs. Here, we aimed to summarize and highlight the current knowledge of the metabolism and transport of silymarin. It was concluded that the major efflux transporters of silybin are multidrug resistance-associated protein (MRP2) and breast cancer resistance protein (BCRP) based on results from the transporter-overexpressing cell lines and MRP2-deficient (TR-) rats. Nevertheless, compounds that inhibit the efflux transporters MRP2 and BCRP can enhance the absorption and activity of silybin. Although silymarin does inhibit certain drug-metabolizing enzymes and drug transporters, such effects are unlikely to manifest in clinical settings. Overall, silymarin is a safe and well-tolerated phytomedicine.
Keywords: drug–drug interaction (DDI); efflux transporters; metabolism; silybin/silymarin.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Rainone F. Milk thistle. Am. Fam. Physician. 2005;72:1285–1288. - PubMed
-
- Lee J.I., Narayan M., Barrett J.S. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;845:95–103. doi: 10.1016/j.jchromb.2006.07.063. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

