Cellular response to bacterial infection in the grasshopper Oxya chinensis
- PMID: 31615769
- PMCID: PMC6826287
- DOI: 10.1242/bio.045864
Cellular response to bacterial infection in the grasshopper Oxya chinensis
Abstract
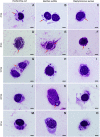
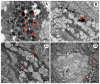
Oxya chinensis is one of the most widespread grasshopper species found in China and one of the most common pests against rice. In view of the importance of haemocytes in insect immunity in general, and the lack of information on the haemocytes of O. chinensis, we examined the haemocytes of this species in detail. We challenged the cellular response of this grasshopper with the bacteria Escherichia coli, Staphylococcus aureus, and Bacillus subtilis Haemocyte morphology was observed using light, scanning electron and transmission electron microscopy, which revealed distinct morphological varieties of haemocytes. Granulocytes and plasmatocytes responded to the bacterial challenge by phagocytosis. Histochemical staining indicated the presence of acid phosphatase in plasmatocytes and granulocytes. We also observed non-phagocytic prohemocytes and vermicytes, but their functions in the circulation are unclear. Insect haemocytes play a crucial role in cellular immunity, and further research is needed for a comprehensive understanding.
Keywords: Haemocyte; Histochemistry; Morphology; Phagocytosis; Wright-Giemsa staining.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures






References
-
- Akai H. and Sato S. (1979). Surface and internal ultrastructure of hemocytes of some insects. In Insect Hemocytes, Development, Forms, Functions and Techniques (ed. Gupta A. P.), pp. 129-154. Cambridge, London: Cambridge University Press.
-
- Anggraeni T., Melanie T. and Putra R. E. (2011). Cellular and humoral immune defenses of Oxya japonica (Orthoptera: Acrididae) to entomopathogenic fungi Metarhizium anisopliae. Entomol. Res. 41, 1-6. 10.1111/j.1748-5967.2010.00311.x - DOI
LinkOut - more resources
Full Text Sources

