Heterogeneity and plasticity of porcine alveolar macrophage and pulmonary interstitial macrophage isolated from healthy pigs in vitro
- PMID: 31615770
- PMCID: PMC6826289
- DOI: 10.1242/bio.046342
Heterogeneity and plasticity of porcine alveolar macrophage and pulmonary interstitial macrophage isolated from healthy pigs in vitro
Abstract
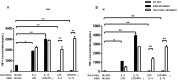
This study investigated the heterogeneity and plasticity of porcine alveolar macrophages (PAM) and pulmonary interstitial macrophages (IM) isolated from healthy pigs, including phenotype, function and gene expression. Dynamic changes of nitric oxide (NO) levels secreted by PAM and IM with stimulation of different doses of lipopolysaccharide (LPS) were investigated by Griess method, and the viability of the PAM and IM cells was investigated by MTT assay. Flow cytometry, fluorescence quantitative PCR and ELISA techniques were used to measure cell phenotype, gene expression and cytokine secretion, respectively. The PAM and IM cells in normal healthy pigs showed heterogeneity with 95.42±1.51% and 31.99±5.84% of CD163+ macrophage, respectively. The NO level in IM was significantly higher versus PAM after LPS treatment. Consistently, the ratio of Arg I/iNOS in IM was much lower than that in PAM, suggesting that the PAM belong to M2 macrophages and the IM belong to M1 macrophages. The PAM and IM cells in normal healthy pigs also showed plasticity. The Arg I/iNOS ratio and TIMP1/MMP12 ratio were significantly decreased in LPS- or LPS+IFNγ-treated PAM and IM, suggesting that cells were polarized towards M1 macrophages under LPS or LPS+IFNγ stimulation. On the contrary, IL-4 and IL-13 stimulation on PAM and IM lead to M2 polarization. A similar result was found in IL-1β gene expression and TNFα secretion. In conclusion, porcine macrophages have shown heterogeneity and plasticity on polarization under the stimulation of LPS, IFNγ, IL-4 and IL-13.
Keywords: Heterogeneity; Plasticity; Porcine alveolar macrophages; Pulmonary interstitial macrophages.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Ait-Ali T., Wilson A. D., Westcott D. G., Clapperton M., Waterfall M., Mellencamp M. A., Drew T. W., Bishop S. C. and Archibald A. L. (2007). Innate immune responses to replication of porcine reproductive and respiratory syndrome virus in isolated swine alveolar macrophages. Viral Immunol. 20, 105-118. 10.1089/vim.2006.0078 - DOI - PubMed
-
- Barroso M. V., Cattani-Cavalieri I., de Brito-Gitirana L., Fautrel A., Lagente V., Schmidt M., Porto L. C., Romana-Souza B., Valença S. S. and Lanzetti M. (2017). Propolis reversed cigarette smoke-induced emphysema through macrophage alternative activation independent of Nrf2. Bioorg. Med. Chem. 25, 5557-5568. 10.1016/j.bmc.2017.08.026 - DOI - PubMed
-
- Bernasconi E., Koutsokera A., Pattaroni C., Dumas D., Camara B., Marsland B. J., Benden C., Pison C., Aubert J. and Nicod L. P. (2015). Correlative changes in macrophage polarization and pulmonary microbiota in lung transplant recipients. J. Heart Lung Transpl. 34, S51 10.1016/j.healun.2015.01.127 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

