Protocol for a multicentre randomised controlled trial evaluating the effects of moderate hypothermia versus normothermia on mortality in patients with refractory cardiogenic shock rescued by venoarterial extracorporeal membrane oxygenation (VA-ECMO) (HYPO-ECMO study)
- PMID: 31615800
- PMCID: PMC6797322
- DOI: 10.1136/bmjopen-2019-031697
Protocol for a multicentre randomised controlled trial evaluating the effects of moderate hypothermia versus normothermia on mortality in patients with refractory cardiogenic shock rescued by venoarterial extracorporeal membrane oxygenation (VA-ECMO) (HYPO-ECMO study)
Abstract
Introduction: Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is widely used to support the most severe forms of cardiogenic shock (CS). Nevertheless, despite extracorporeal membrane oxygenation (ECMO) use, mortality still remains high (50%). Moderate hypothermia (MH) (33°C-34°C) may improve cardiac performance and decrease ischaemia-reperfusion injuries. The use of MH during VA-ECMO is strongly supported by experimental and preliminary clinical data.
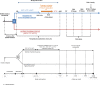
Methods and analysis: The Hypothermia-Extracorporeal Membrane Oxygenation (HYPO-ECMO) study is a multicentre, prospective, controlled randomised trial between an MH group (33°C≤T°C≤34°C) and normothermia group (36°C≤T°C≤37°C). The primary endpoint is all-cause mortality at day 30 following randomisation. The study will also assess as secondary endpoints the effects of targeted temperature management strategies on (1) mortality rate at different time points, (2) organ failure and supportive treatment use and (3) safety. All intubated adults with refractory CS supported with VA-ECMO will be screened. Exclusion criteria are patients having undergone cardiac surgery for heart transplantation or left or biventricular assist device implantation, acute poisoning with cardiotoxic drugs, pregnancy, uncontrolled bleeding and refractory cardiac arrest.Three-hundred and thirty-four patients will be randomised and followed up to 6 months to detect a 15% difference in mortality. Data analysis will be intention to treat. The differences between the two study groups in the risk of all-cause mortality at day 30 following randomisation will be studied using logistic regression analysis adjusted for postcardiotomy setting, prior cardiac arrest, prior myocardial infarction, age, vasopressor dose, Sepsis-related Organ Failure Assessment (SOFA) score and lactate at randomisation.
Ethics and dissemination: Ethics approval has been granted by the Comité de Protection des Personnes Est III Ethics Committee. The trial has been approved by the French Health Authorities (Agence Nationale de la Sécurité du Médicament et des Produits de Santé). Dissemination of results will be performed via journal articles and presentations at national and international conferences. Since this study is also the first step in the constitution of an 'ECMO Trials Group', its results will also be disseminated by the aforementioned group.
Trial registration number: NCT02754193.
Keywords: ECMO VA; cardiogenic shock; hypothermia.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Nielsen N, Wetterslev J, Cronberg T, et al. . Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2013;369:2197–206. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials