Immune Cell PD-L1 Colocalizes with Macrophages and Is Associated with Outcome in PD-1 Pathway Blockade Therapy
- PMID: 31615933
- PMCID: PMC7024671
- DOI: 10.1158/1078-0432.CCR-19-1040
Immune Cell PD-L1 Colocalizes with Macrophages and Is Associated with Outcome in PD-1 Pathway Blockade Therapy
Abstract
Purpose: Programmed death ligand 1 (PD-L1) is expressed in tumor cells and immune cells, and both have been associated with response to anti-PD-1 axis immunotherapy. Here, we examine the expression of PD-L1 to determine which cell type carries the predictive value of the test.
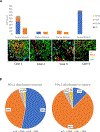
Experimental design: We measured the expression of PD-L1 in multiple immune cells with two platforms and confocal microscopy on three retrospective Yale NSCLC cohorts (425 nonimmunotherapy-treated cases and 62 pembrolizumab/nivolumab/atezolizumab-treated cases). The PD-L1 level was selectively measured in different immune cell subsets using two multiplexed quantitative immunofluorescence panels, including CD56 for natural killer cells, CD68 for macrophages, and CD8 for cytotoxic T cells.
Results: PD-L1 was significantly higher in macrophages in both tumor and stromal compartment compared with other immune cells. Elevated PD-L1 in macrophages was correlated with high PD-L1 level in tumor as well as CD8 and CD68 level (P < 0.0001). High PD-L1 expression in macrophages was correlated with better overall survival (OS; P = 0.036 by cell count/P = 0.019 by molecular colocalization), while high PD-L1 expression in tumor cells was not.
Conclusions: In nearly 500 non-small cell lung cancer (NSCLC) cases, the predominant immune cell type that expresses PD-L1 is CD68+ macrophages. The level of PD-L1 in macrophages is significantly associated with the level of PD-L1 in tumor cells and infiltration by CD8+ T cells, suggesting a connection between high PD-L1 and "hot" tumors. In anti-PD-1 axis therapy-treated patients, high levels of PD-L1 expression in macrophages are associated with longer OS and may be responsible for the predictive effect of the marker.
©2019 American Association for Cancer Research.
Conflict of interest statement
Possible Conflict of Interest
Kurt Schalper has served as a consultant, advisor or served on a Scientific Advisory Board for Celgene, Moderna Therapeutics Shattuck Labs, Astra Zeneca, Pierre-Fabre and Abbvie. He has received research funding from Genoptix/Navigate (Novartis), Vasculox/Tioma, Tesaro, Moderna Therapeutics, Tesaro Pharmaceuticals, Surface Oncology, Pierre-Fabre Research Institute, Merck and Bristol-Myers Squibb.
David Rimm has served as a consultant, advisor or served on a Scientific Advisory Board for Amgen, Astra Zeneca, Agendia, Biocept, BMS, Cell Signaling Technology, Cepheid, Daiichi Sankyo, GSK, Merck, NanoString, Perkin Elmer, PAIGE, and Ultivue. He has received research funding from Astra Zeneca, Cepheid, Nanostring, Navigate/Novartis, NextCure, Lilly, Ultivue, and Perkin Elmer
Jon Zugazagoitia has received consulting honoraria from Guardant Health.
Figures

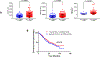
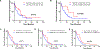
References
-
- Sharpe AH, Wherry EJ, Ahmed R, et al.: The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8:239–45, 2007 - PubMed
-
- Dong H, Strome SE, Salomao DR, et al.: Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

