Antibiotics and Host-Tailored Probiotics Similarly Modulate Effects on the Developing Avian Microbiome, Mycobiome, and Host Gene Expression
- PMID: 31615957
- PMCID: PMC6794479
- DOI: 10.1128/mBio.02171-19
Antibiotics and Host-Tailored Probiotics Similarly Modulate Effects on the Developing Avian Microbiome, Mycobiome, and Host Gene Expression
Abstract
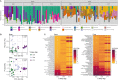
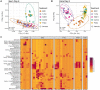
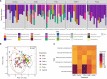
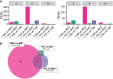
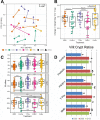
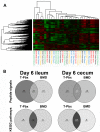
The microbiome is important to all animals, including poultry, playing a critical role in health and performance. Low-dose antibiotics have historically been used to modulate food production animals and their microbiome. Identifying alternatives to antibiotics conferring similar modulatory properties has been elusive. The purpose of this study was to determine if a host-tailored probiotic could recapitulate effects of a low-dose antibiotic on host response and the developing microbiome. Over 13 days of life, turkey poults were supplemented continuously with a low-dose antibiotic or oral supplementation of a prebiotic with or without two different probiotics (8 cage units, n = 80 per group). Gastrointestinal bacterial and fungal communities of poults were characterized by 16S rRNA gene and ITS2 amplicon sequencing. Localized and systemic host gene expression was assessed using transcriptome sequencing (RNA-Seq), kinase activity was assessed by avian-specific kinome peptide arrays, and performance parameters were assessed. We found that development of the early-life microbiome of turkey poults was tightly ordered in a tissue- and time-specific manner. Low-dose antibiotic and turkey-tailored probiotic supplementation, but not nontailored probiotic supplementation, elicited similar shifts in overall microbiome composition during development compared to controls. Treatment-induced bacterial changes were accompanied by parallel shifts in the fungal community and host gene expression and enhanced performance metrics. These results were validated in pen trials that identified further additive effects of the turkey-tailored probiotic combined with different prebiotics. Alternative approaches to low-dose antibiotic use in poultry are feasible and can be optimized utilizing the indigenous poultry microbiome. Similar approaches may also be beneficial for humans.IMPORTANCE Alternative approaches are greatly needed to reduce the need for antibiotic use in food animal production. This study utilized a pipeline for the development of a host-tailored probiotic to enhance performance in commercial turkeys and modulate their microbiota, similar to the effects of low-dose antibiotic administration. We determined that a host-tailored probiotic, developed in the context of the commercial turkey gut microbiome, was more effective at modulating these parameters than a nontailored probiotic cocktail. Furthermore, the host-tailored probiotic mimicked many of the effects of a low-dose antibiotic growth promoter. Surprisingly, the effects of the antibiotic growth promoter and host-tailored probiotic were observed across kingdoms, illustrating the coordinated interkingdom effects of these approaches. This work suggests that tailored approaches to probiotic development hold promise for modulating the avian host and its microbiota.
Keywords: antibiotic; bacteria; fungi; host; microbiota; poultry; probiotics.
Copyright © 2019 Ward et al.
Figures


References
-
- National Agricultural Statistics Service. May 2019. Poultry—production and value. 2018 summary. National Agricultural Statistics Service, United States Department of Agriculture, Washington, DC: https://www.nass.usda.gov/Publications/Todays_Reports/reports/plva0519.pdf. Accessed 12 August 2019.
-
- Danzeisen JL, Clayton JB, Huang H, Knights D, McComb B, Hayer SS, Johnson TJ. 2015. Temporal relationships exist between cecum, ileum, and litter bacterial microbiomes in a commercial turkey flock, and subtherapeutic penicillin treatment impacts ileum bacterial community establishment. Front Vet Sci 2:56. doi:10.3389/fvets.2015.00056. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

