Probiotic Yeasts Inhibit Virulence of Non -albicans Candida Species
- PMID: 31615960
- PMCID: PMC6794482
- DOI: 10.1128/mBio.02307-19
Probiotic Yeasts Inhibit Virulence of Non -albicans Candida Species
Abstract
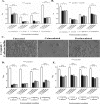
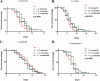
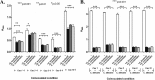
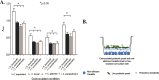
Systemic infections of Candida species pose a significant threat to public health. Toxicity associated with current therapies and emergence of resistant strains present major therapeutic challenges. Here, we report exploitation of the probiotic properties of two novel, food-derived yeasts, Saccharomyces cerevisiae (strain KTP) and Issatchenkia occidentalis (strain ApC), as an alternative approach to combat widespread opportunistic fungal infections. Both yeasts inhibit virulence traits such as adhesion, filamentation, and biofilm formation of several non-albicans Candida species, including Candida tropicalis, Candida krusei, Candida glabrata, and Candida parapsilosis as well as the recently identified multidrug-resistant species Candida auris They inhibit adhesion to abiotic surfaces as well as cultured colon epithelial cells. Furthermore, probiotic treatment blocks the formation of biofilms of individual non-albicans Candida strains as well as mixed-culture biofilms of each non-albicans Candida strain in combination with Candida albicans The probiotic yeasts attenuated non-albicans Candida infections in a live animal. In vivo studies using Caenorhabditis elegans suggest that exposure to probiotic yeasts protects nematodes from infection with non-albicans Candida strains compared to worms that were not exposed to the probiotic yeasts. Furthermore, application of probiotic yeasts postinfection with non-albicans Candida alleviated pathogenic colonization of the nematode gut. The probiotic properties of these novel yeasts are better than or comparable to those of the commercially available probiotic yeast Saccharomyces boulardii, which was used as a reference strain throughout this study. These results indicate that yeasts derived from food sources could serve as an effective alternative to antifungal therapy against emerging pathogenic Candida species.IMPORTANCE Non-albicans Candida-associated infections have emerged as a major risk factor in the hospitalized and immunecompromised patients. Besides, antifungal-associated complications occur more frequently with these non-albicans Candida species than with C. albicans Therefore, as an alternative approach to combat these widespread non-albicans Candida-associated infections, here we showed the probiotic effect of two yeasts, Saccharomyces cerevisiae (strain KTP) and Issatchenkia occidentalis (ApC), in preventing adhesion and biofilm formation of five non-albicans Candida strains, Candida tropicalis, Candida krusei, Candida glabrata, Candida parapsilosis, and Candida auris The result would influence the current trend of the conversion of conventional antimicrobial therapy into beneficial probiotic microbe-associated antimicrobial treatment.
Keywords: Caco-2 cell monolayer; Caenorhabditis elegans; Candida albicans; Candida auris; Candida glabrata; Candida krusei; Candida parapsilosis; Candida tropicalis; biofilm; mixed-species Candida biofilm; plastic adhesion assay; probiotic yeasts.
Copyright © 2019 Kunyeit et al.
Figures


References
-
- Rao B. 2008. Emerging pathogens of the Candida species In Sandai D. (ed), Candida albicans. IntechOpen, London, United Kingdom. doi:10.5772/intechopen.80378. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

