Skeletal Muscle Atrophy Was Alleviated by Salidroside Through Suppressing Oxidative Stress and Inflammation During Denervation
- PMID: 31616291
- PMCID: PMC6763704
- DOI: 10.3389/fphar.2019.00997
Skeletal Muscle Atrophy Was Alleviated by Salidroside Through Suppressing Oxidative Stress and Inflammation During Denervation
Abstract
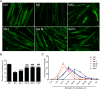
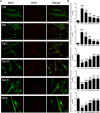
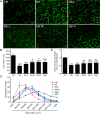
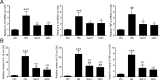
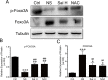
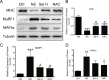
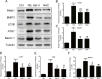
Skeletal muscle atrophy is a common and debilitating condition that lacks an effective therapy. Oxidative stress and inflammation are two main molecular mechanisms involved in muscle atrophy. In the current study, we want to explore whether and how salidroside, with antioxidant and anti-inflammatory properties, protects against skeletal muscle atrophy induced by denervation. First, oxidative stress and inflammatory response were examined during myotube atrophy induced by nutrition deprivation. The results demonstrated that oxidative stress and inflammatory response were induced in cultured myotubes suffered from nutrition deprivation, and salidroside not only inhibited oxidative stress and inflammatory response but also attenuated nutrition deprivation-induced myotube atrophy, as evidenced by an increased myotube diameter. The antioxidant, anti-inflammatory, and antiatrophic properties of salidroside in cultured myotubes were confirmed in denervated mouse models. The mice treated with salidroside showed less oxidative stress and less inflammatory cytokines, as well as higher skeletal muscle wet weight ratio and larger average cross sectional areas of myofibers compared with those treated with saline only during denervation-induced skeletal muscle atrophy. Moreover, salidroside treatment of denervated mice resulted in an inhibition of the activation of mitophagy in skeletal muscle. Furthermore, salidroside reduced the expression of atrophic genes, including MuRF1 and MAFbx, autophagy genes, including PINK1, BNIP3, LC3B, ATG7, and Beclin1, and transcription factor forkhead box O3 A (Foxo3A), and improved the expression of myosin heavy chain and transcriptional factor phosphorylated Foxo3A. Taken together, these results suggested that salidroside alleviated denervation-induced muscle atrophy by suppressing oxidative stress and inflammation.
Keywords: inflammation; muscle atrophy; nerve injury; oxidative stress; salidroside.
Copyright © 2019 Huang, Fang, Ma, Zhang, Qiu, Gu, Yang and Sun.
Figures
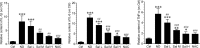



References
LinkOut - more resources
Full Text Sources
Research Materials

