Carbapenem-Resistant Acinetobacter baumannii in Three Tertiary Care Hospitals in Mexico: Virulence Profiles, Innate Immune Response and Clonal Dissemination
- PMID: 31616391
- PMCID: PMC6764332
- DOI: 10.3389/fmicb.2019.02116
Carbapenem-Resistant Acinetobacter baumannii in Three Tertiary Care Hospitals in Mexico: Virulence Profiles, Innate Immune Response and Clonal Dissemination
Abstract
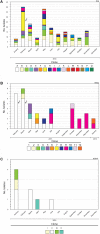
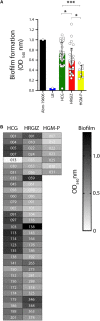
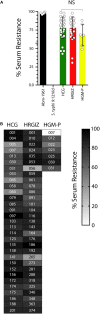
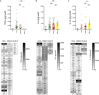
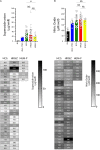
Acinetobacter baumannii is one of the most important nosocomial pathogens distributed worldwide. Due to its multidrug-resistance and the propensity for the epidemic spread, the World Health Organization includes this bacterium as a priority health issue for development of new antibiotics. The aims of this study were to investigate the antimicrobial resistance profile, the clonal relatedness, the virulence profiles, the innate host immune response and the clonal dissemination of A. baumannii in Hospital Civil de Guadalajara (HCG), Hospital Regional General Ignacio Zaragoza (HRGIZ) and Pediatric ward of the Hospital General de México Eduardo Liceaga (HGM-P). A total of 252 A. baumannii clinical isolates were collected from patients with nosocomial infections in these hospitals between 2015 and 2016. These isolates showed a multidrug-resistant profile and most of them only susceptible to colistin. Furthermore, 83.3 and 36.9% of the isolates carried the bla OXA- 24 and bla TEM- 1 genes for resistance to carbapenems and β-lactam antibiotics, respectively. The clonal relatedness assessed by pulsed-field gel electrophoresis (PFGE) and by multi-locus sequence typing (MLST) demonstrated a genetic diversity. Remarkably, the ST136, ST208 and ST369 that belonged to the clonal complex CC92 and ST758 and ST1054 to the CC636 clonal complex were identified. The ST136 was a high-risk persistent clone involved in an outbreak at HCG and ST369 were related to the first carbapenem-resistant A. baumannii outbreak in HRGIZ. Up to 58% isolates were able to attach to A549 epithelial cells and 14.5% of them induced >50% of cytotoxicity. A549 cells infected with A. baumannii produced TNFα, IL-6 and IL-1β and the oxygen and nitrogen reactive species that contributes to the development of an inflammatory immune response. Up to 91.3% of clinical isolates were resistant to normal human serum activity. Finally, 98.5% of the clinical isolates were able to form biofilm over polystyrene tubes. In summary, these results demonstrate the increasingly dissemination of multidrug-resistant A. baumannii clones in three hospitals in Mexico carrying diverse bacterial virulence factors that could contribute to establishment of the innate immune response associated to the fatality risks in seriously ill patients.
Keywords: Acinetobacter baumannii; MLST; Mexico; adherence/invasion; biofilm; clonal dissemination; immune response; multidrug resistance.
Copyright © 2019 Alcántar-Curiel, Rosales-Reyes, Jarillo-Quijada, Gayosso-Vázquez, Fernández-Vázquez, Toledano-Tableros, Giono-Cerezo, Garza-Villafuerte, López-Huerta, Vences-Vences, Morfín-Otero, Rodríguez-Noriega, López-Álvarez, Espinosa-Sotero and Santos-Preciado.
Figures



References
-
- Alcántar-Curiel M. D., Fernández-Vázquez J. L., Toledano-Tableros J. E., Gayosso-Vázquez C., Jarillo-Quijada M. D., López-Álvarez M. D. R., et al. (2019). Emergence of IncFIA plasmid-carrying blaNDM-1 among Klebsiella pneumoniae and Enterobacter cloacae isolates in a Tertiary Referral Hospital in Mexico. Microb. Drug Resist. 25 830–838. 10.1089/mdr.2018.0306 - DOI - PubMed
-
- Alcántar-Curiel M. D., García-Torres L. F., González-Chávez M. I., Morfín-Otero R., Gayosso-Vázquez C., Jarillo-Quijada M. D., et al. (2014). Molecular mechanisms associated with nosocomial carbapenem-resistant Acinetobacter baumannii in Mexico. Arch. Med. Res. 45 553–560. 10.1016/j.arcmed.2014.10.006 - DOI - PubMed
-
- Ambrosi C., Scribano D., Aleandri M., Zagaglia C., Di Francesco L., Putignani L., et al. (2017). Acinetobacter baumannii virulence traits: a comparative study of a novel sequence type with other italian endemic international clones. Front. Microbiol. 8:1977. 10.3389/fmicb.2017.01977 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

