Imaging Distal Aqueous Outflow Pathways in a Spontaneous Model of Congenital Glaucoma
- PMID: 31616579
- PMCID: PMC6788461
- DOI: 10.1167/tvst.8.5.22
Imaging Distal Aqueous Outflow Pathways in a Spontaneous Model of Congenital Glaucoma
Abstract
Purpose: To validate the use of aqueous angiography (AA) in characterizing distal aqueous outflow pathways in normal and glaucomatous cats.
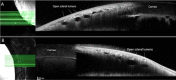
Methods: Ex vivo AA and optical coherence tomography (OCT) were performed in nine adult cat eyes (5 feline congenital glaucoma [FCG] and 4 normal), following intracameral infusion of 2.5% fluorescein and/or 0.4% indocyanine green (ICG) at physiologic intraocular pressure (IOP). Scleral OCT line scans were acquired in areas of high- and low-angiographic signal. Tissues dissected in regions of high- and low-AA signal, were sectioned and hematoxylin and eosin (H&E)-stained or immunolabeled (IF) for vascular endothelial and perivascular cell markers. Outflow vessel numbers and locations were compared between groups by Student's t-test.
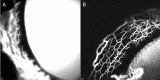
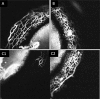
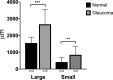
Results: AA yielded circumferential, high-quality images of distal aqueous outflow pathways in normal and FCG eyes. No AA signal or scleral lumens were appreciated in one buphthalmic FCG eye, though collapsed vascular profiles were identified on IF. The remaining eight of nine eyes all showed segmental AA signal, distinguished by differences in time of signal onset. AA signal always corresponded with lumens seen on OCT. Numbers of intrascleral vessels were not significantly different between groups, but scleral vessels were significantly more posteriorly located relative to the limbus in FCG.
Conclusions: A capacity for distal aqueous humor outflow was confirmed by AA in FCG eyes ex vivo but with significant posterior displacement of intrascleral vessels relative to the limbus in FCG compared with normal eyes.
Translational relevance: This report provides histopathologic correlates of advanced diagnostic imaging findings in a spontaneous model of congenital glaucoma.
Keywords: aqueous angiography; glaucoma anterior segment; imaging; optical coherence tomography.
Copyright 2019 The Authors.
Figures






References
-
- Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS. 1999;3:26–32. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

