Low-Cost Ru/C-Catalyzed Depolymerization of the Polymeric Proanthocyanidin-Rich Fraction from Bark To Produce Oligomeric Proanthocyanidins with Antioxidant Activity
- PMID: 31616825
- PMCID: PMC6787890
- DOI: 10.1021/acsomega.9b02071
Low-Cost Ru/C-Catalyzed Depolymerization of the Polymeric Proanthocyanidin-Rich Fraction from Bark To Produce Oligomeric Proanthocyanidins with Antioxidant Activity
Abstract
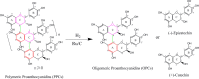
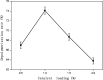
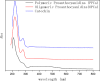
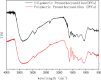
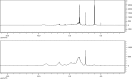
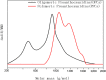
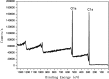
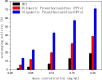
A new method has been developed for the high-value utilization of larch bark, which is regarded as a low-value byproduct of the logging industry. Polymeric proanthocyanidins (PPCs) were extracted from the Larix gmelinii bark and depolymerized by catalytic hydrogenolysis, using ruthenium/carbon (Ru/C) as the catalyst. The method has been found that although the molecular weight of the depolymerized product was significantly lower, the basic structural units were not destroyed, and the product retained a condensed flavanol polyphenol structure; the depolymerized product contains very little Ru metal and thus complies with food safety standards; the antioxidant properties of both the depolymerized products and PPCs were better than those of the commonly used antioxidant 2,6-di-tert-butyl-4-methylphenol. The relative molecular weight and steric hindrance of the depolymerized products were lower than those of the PPCs, leading to better antioxidant performance. A new technical route for the depolymerization of PPCs from the L. gmelinii bark is provided. The route offers practical and commercial advantages, and the product could have many applications as an antioxidant.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- An L.; Si C.; Wang G.; Sui W.; Tao Z. Enhancing the solubility and antioxidant activity of high-molecular-weight lignin by moderate depolymerization via in situ ethanol/acid catalysis. Ind. Crops Prod. 2019, 128, 177–185. 10.1016/j.indcrop.2018.11.009. - DOI
-
- Tudor E. M.; Barbu M. C.; Petutschnigg A.; Réh R. Added-value for wood bark as a coating layer for flooring tiles. J. Cleaner Prod. 2018, 170, 1354–1360. 10.1016/j.jclepro.2017.09.156. - DOI
-
- Fraser K.; Harrison S. J.; Lane G. A.; Otter D. E.; Hemar Y.; Quek S.-Y.; Rasmussen S. HPLC–MS/MS profiling of proanthocyanidins in teas: A comparative study. J. Food Compos. Anal. 2012, 26, 43–51. 10.1016/j.jfca.2012.01.004. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
