4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration
- PMID: 31616982
- PMCID: PMC7192393
- DOI: 10.1007/s00401-019-02080-2
4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration
Erratum in
-
Correction to: 4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration.Acta Neuropathol. 2020 Jan;139(1):79-81. doi: 10.1007/s00401-019-02092-y. Acta Neuropathol. 2020. PMID: 31748840 Free PMC article.
Abstract
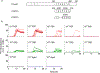
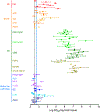
To address the need for more meaningful biomarkers of tauopathies, we have developed an ultrasensitive tau seed amplification assay (4R RT-QuIC) for the 4-repeat (4R) tau aggregates of progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and other diseases with 4R tauopathy. The assay detected seeds in 106-109-fold dilutions of 4R tauopathy brain tissue but was orders of magnitude less responsive to brain with other types of tauopathy, such as from Alzheimer's disease cases. The analytical sensitivity for synthetic 4R tau fibrils was ~ 50 fM or 2 fg/sample. A novel dimension of this tau RT-QuIC testing was the identification of three disease-associated classes of 4R tau seeds; these classes were revealed by conformational variations in the in vitro amplified tau fibrils as detected by thioflavin T fluorescence amplitudes and FTIR spectroscopy. Tau seeds were detected in postmortem cerebrospinal fluid (CSF) from all neuropathologically confirmed PSP and CBD cases but not in controls. CSF from living subjects had weaker seeding activities; however, mean assay responses for cases clinically diagnosed as PSP and CBD/corticobasal syndrome were significantly higher than those from control cases. Altogether, 4R RT-QuIC provides a practical cell-free method of detecting and subtyping pathologic 4R tau aggregates as biomarkers.
Keywords: Biomarker; Corticobasal degeneration; Diagnosis; Progressive supranuclear palsy; Strain; Tau.
Figures



References
-
- Alster P, Nieciecki M, Koziorowski DM, Cacko A, Charzynska I, Krolicki L et al. (2019) Thalamic and cerebellar hypoperfusion in single photon emission computed tomography may differentiate multiple system atrophy and progressive supranuclear palsy. Medicine (Baltimore) 98:e16603 10.1097/MD.0000000000016603 - DOI - PMC - PubMed
-
- Baron GS, Hughson AG, Raymond GJ, Offerdahl DK, Barton KA, Raymond LD et al. (2011) Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra. Biochemistry 50:4479–4490. 10.1021/bi2003907 - DOI - PMC - PubMed
-
- Bessen RA, Kocisko DA, Raymond GJ, Nandan S, Lansbury PT Jr, Caughey B (1995) Nongenetic propagation of strain-specific phenotypes of scrapie prion protein. Nature 375:698–700 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

