Levo-corydalmine Attenuates Vincristine-Induced Neuropathic Pain in Mice by Upregulating the Nrf2/HO-1/CO Pathway to Inhibit Connexin 43 Expression
- PMID: 31617070
- PMCID: PMC7007458
- DOI: 10.1007/s13311-019-00784-7
Levo-corydalmine Attenuates Vincristine-Induced Neuropathic Pain in Mice by Upregulating the Nrf2/HO-1/CO Pathway to Inhibit Connexin 43 Expression
Abstract
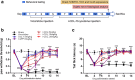
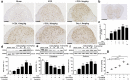
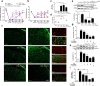
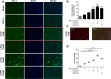
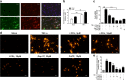
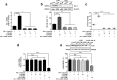
Antimicrotubulin chemotherapeutic agents, including plant-derived vincaalkaloids such as vincristine, can cause peripheral neuropathic pain. Exogenously activated heme oxygenase 1 (HO-1) is a potential therapy for chemotherapy-induced neuroinflammation. In this study, we investigated a role for Nrf2/HO-1/CO in mediating vincristine-induced neuroinflammation by inhibiting connexin 43 (Cx43) production in the spinal cord following the intrathecal application of the HO-1 inducer protoporphyrin IX cobalt chloride (CoPP) or inhibitor protoporphyrin IX zinc (ZnPP), and we analyzed the underlying mechanisms by which levo-corydalmine (l-CDL, a tetrahydroprotoberberine) attenuates vincristine-induced pain. Treatment with levo-corydalmine or oxycodone hydrochloride (a semisynthetic opioid analgesic, used as a positive control) attenuated vincristine-induced persistent pain hypersensitivity and degeneration of the sciatic nerve. In addition, the increased prevalence of atypical mitochondria induced by vincristine was ameliorated by l-CDL in both A-fibers and C-fibers. Next, we evaluated whether nuclear factor E2-related factor 2 (Nrf2), an upstream activator of HO-1, directly bound to the HO-1 promoter sequence and degraded heme to produce carbon monoxide (CO) following stimulation with vincristine. Notably, l-CDL dose-dependently increased HO-1/CO expression by activating Nrf2 to inhibit Cx43 expression in both the spinal cord and in cultured astrocytes stimulated with TNF-α, corresponding to decreased Cx43-mediated hemichannel. Furthermore, l-CDL had no effect on Cx43 following the silencing of the HO-1 gene. Taken together, our findings reveal a novel mechanism by which Nrf2/HO-1/CO mediates Cx43 expression in vincristine-induced neuropathic pain. In addition, the present findings suggest that l-CDL likely protects against nerve damage and attenuates vincristine-induced neuroinflammation by upregulating Nrf2/HO-1/CO to inhibit Cx43 expression.
Keywords: Vincristine; connexin-43; heme oxygenase 1; neuropathic pain; nuclear factor E2-related factor 2.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Levo-corydalmine alleviates vincristine-induced neuropathic pain in mice by inhibiting an NF-kappa B-dependent CXCL1/CXCR2 signaling pathway.Neuropharmacology. 2018 Jun;135:34-47. doi: 10.1016/j.neuropharm.2018.03.004. Epub 2018 Mar 5. Neuropharmacology. 2018. PMID: 29518397
-
Exogenous induction of HO-1 alleviates vincristine-induced neuropathic pain by reducing spinal glial activation in mice.Neurobiol Dis. 2015 Jul;79:100-10. doi: 10.1016/j.nbd.2015.04.012. Epub 2015 May 6. Neurobiol Dis. 2015. PMID: 25956228
-
Levo-corydalmine attenuates microglia activation and neuropathic pain by suppressing ASK1-p38 MAPK/NF-κB signaling pathways in rat spinal cord.Reg Anesth Pain Med. 2020 Mar;45(3):219-229. doi: 10.1136/rapm-2019-100875. Epub 2020 Jan 2. Reg Anesth Pain Med. 2020. PMID: 31898581
-
Carbon Monoxide-Releasing Molecule-2 Inhibits Connexin 43-Hemichannel Activity in Spinal Cord Astrocytes to Attenuate Neuropathic Pain.J Mol Neurosci. 2017 Sep;63(1):58-69. doi: 10.1007/s12031-017-0957-2. Epub 2017 Aug 6. J Mol Neurosci. 2017. PMID: 28780624
-
Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice.PLoS One. 2012;7(8):e43693. doi: 10.1371/journal.pone.0043693. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22928017 Free PMC article.
Cited by
-
Resolvin D1/N-formyl peptide receptor 2 ameliorates paclitaxel-induced neuropathic pain through the activation of IL-10/Nrf2/HO-1 pathway in mice.Front Immunol. 2023 Mar 13;14:1091753. doi: 10.3389/fimmu.2023.1091753. eCollection 2023. Front Immunol. 2023. PMID: 36993950 Free PMC article.
-
Effects of nicorandil on p120 expression in the spinal cord and dorsal root ganglion of rats with chronic postsurgical pain.Mol Med Rep. 2020 Dec;22(6):4821-4827. doi: 10.3892/mmr.2020.11546. Epub 2020 Sep 28. Mol Med Rep. 2020. PMID: 33173987 Free PMC article.
-
The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) Activation in Preclinical Models of Peripheral Neuropathic Pain.Antioxidants (Basel). 2022 Feb 21;11(2):430. doi: 10.3390/antiox11020430. Antioxidants (Basel). 2022. PMID: 35204312 Free PMC article. Review.
-
Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment.Oncol Ther. 2021 Dec;9(2):385-450. doi: 10.1007/s40487-021-00168-y. Epub 2021 Oct 16. Oncol Ther. 2021. PMID: 34655433 Free PMC article. Review.
-
Neurodegeneration in Multiple Sclerosis: The Role of Nrf2-Dependent Pathways.Antioxidants (Basel). 2022 Jun 10;11(6):1146. doi: 10.3390/antiox11061146. Antioxidants (Basel). 2022. PMID: 35740042 Free PMC article. Review.
References
-
- Jackson DV, Jr, Castle MC, Poplack DG, Bender RA. Pharmacokinetics of vincristine in the cerebrospinal fluid of subhuman primates. Cancer Res. 1980;40(3):722–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

