Bimodal Actions of a Naphthyridone/Aminopiperidine-Based Antibacterial That Targets Gyrase and Topoisomerase IV
- PMID: 31617352
- PMCID: PMC7450530
- DOI: 10.1021/acs.biochem.9b00805
Bimodal Actions of a Naphthyridone/Aminopiperidine-Based Antibacterial That Targets Gyrase and Topoisomerase IV
Abstract
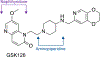
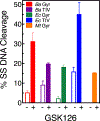
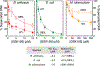
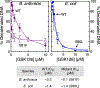
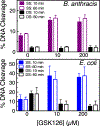
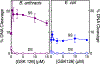
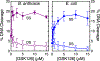
Gyrase and topoisomerase IV are the targets of fluoroquinolone antibacterials. However, the rise in antimicrobial resistance has undermined the clinical use of this important drug class. Therefore, it is critical to identify new agents that maintain activity against fluoroquinolone-resistant strains. One approach is to develop non-fluoroquinolone drugs that also target gyrase and topoisomerase IV but interact differently with the enzymes. This has led to the development of the "novel bacterial topoisomerase inhibitor" (NBTI) class of antibacterials. Despite the clinical potential of NBTIs, there is a relative paucity of data describing their mechanism of action against bacterial type II topoisomerases. Consequently, we characterized the activity of GSK126, a naphthyridone/aminopiperidine-based NBTI, against a variety of Gram-positive and Gram-negative bacterial type II topoisomerases, including gyrase from Mycobacterium tuberculosis and gyrase and topoisomerase IV from Bacillus anthracis and Escherichia coli. GSK126 enhanced single-stranded DNA cleavage and suppressed double-stranded cleavage mediated by these enzymes. It was also a potent inhibitor of gyrase-catalyzed DNA supercoiling and topoisomerase IV-catalyzed decatenation. Thus, GSK126 displays a similar bimodal mechanism of action across a variety of species. In contrast, GSK126 displayed a variable ability to overcome fluoroquinolone resistance mutations across these same species. Our results suggest that NBTIs elicit their antibacterial effects by two different mechanisms: inhibition of gyrase/topoisomerase IV catalytic activity or enhancement of enzyme-mediated DNA cleavage. Furthermore, the relative importance of these two mechanisms appears to differ from species to species. Therefore, we propose that the mechanistic basis for the antibacterial properties of NBTIs is bimodal in nature.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


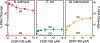
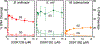

References
-
- Levine C, Hiasa H, and Marians KJ (1998) DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim. Biophys. Acta 1400, 29–43. - PubMed
-
- Wang JC (2002) Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol 3, 430–440. - PubMed
-
- Chen SH, Chan NL, and Hsieh TS (2013) New mechanistic and functional insights into DNA topoisomerases. Annu. Rev. Biochem 82, 139–170. - PubMed
-
- Hooper DC (2001) Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin. Infect. Dis 32 Suppl. 1, S9–S15. - PubMed
-
- Andriole VT (2005) The quinolones: past, present, and future. Clin. Infect. Dis 41 Suppl. 2, S113–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

