Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors
- PMID: 31617564
- PMCID: PMC8109411
- DOI: 10.1093/annonc/mdz410
Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors
Abstract
Background: Advances in diagnostic and therapeutic strategies in oncology have significantly increased the chance of survival of cancer patients, even those with metastatic disease. However, cancer-related cognitive impairment (CRCI) is frequently reported in patients treated for non-central nervous system cancers, particularly during and after chemotherapy.
Design: This review provides an update of the state of the art based on PubMed searches between 2012 and March 2019 on 'cognition', 'cancer', 'antineoplastic agents' or 'chemotherapy'. It includes the most recent clinical, imaging and pre-clinical data and reports management strategies of CRCI.
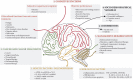
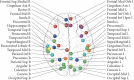
Results: Evidence obtained primarily from studies on breast cancer patients highlight memory, processing speed, attention and executive functions as the most cognitive domains impaired post-chemotherapy. Recent investigations established that other cancer treatments, such as hormone therapies and targeted therapies, can also induce cognitive deficits. Knowledge regarding predisposing factors, biological markers or brain functions associated with CRCI has improved. Factors such as age and genetic polymorphisms of apolipoprotein E, catechol-O-methyltransferase and BDNF may predispose individuals to a higher risk of cognitive impairment. Poor performance on neuropsychological tests were associated with volume reduction in grey matter, less connectivity and activation after chemotherapy. In animals, hippocampus-based memory and executive functions, mediated by the frontal lobes, were shown to be particularly susceptible to the effects of chemotherapy. It involves altered neurogenesis, mitochondrial dysfunction or brain cytokine response. An important next step is to identify strategies for managing cognitive difficulties, with primary studies to assess cognitive training and physical exercise regimens.
Conclusions: CRCI is not limited to chemotherapy. A multidisciplinary approach has improved our knowledge of the complex mechanisms involved. Nowadays, studies evaluating cognitive rehabilitation programmes are encouraged to help patients cope with cognitive difficulties and improve quality of life during and after cancer.
Keywords: managementof cognitive impairment; animal model; cancer patients; cancer treatments; cancer-related cognitive impairment; neuro-imaging.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Joly F., Giffard B., Rigal O. Impact of cancer and its treatments on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage. 2015;50(6):830–841. - PubMed
-
- Wefel J.S., Vardy J., Ahles T., Schagen S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

