Engineering a single-chain antibody against Trypanosoma cruzi metacyclic trypomastigotes to block cell invasion
- PMID: 31618282
- PMCID: PMC6795462
- DOI: 10.1371/journal.pone.0223773
Engineering a single-chain antibody against Trypanosoma cruzi metacyclic trypomastigotes to block cell invasion
Abstract
Trypanosoma cruzi is a flagellate protozoan pathogen that causes Chagas disease. Currently there is no preventive treatment and the efficiency of the two drugs available is limited to the acute phase. Therefore, there is an unmet need for innovative tools to block transmission in endemic areas. In this study, we engineered a novel recombinant molecule able to adhere to the T. cruzi surface, termed scFv-10D8, that consists of a single-chain variable fragment (scFv) derived from mAb-10D8 that targets gp35/50. The synthetic gene encoding scFv-10D8 was cloned and fused to a 6×His tag and expressed in a prokaryotic expression system. Total periplasmic or 6xHis tag affinity-purified fractions of scFv-10D8 retained the capacity to bind to gp35/50, as shown by Western blot analyses. Pre-incubation of metacyclic trypomastigotes with scFv-10D8 showed a remarkable reduction in cell invasion capacity. Our results suggest that scFv-10D8 can be used in a paratransgenic approach to target parasites in insect vectors, avoiding dissemination of infective forms. Such advances in the development of this functional molecule will surely prompt the improvement of alternative strategies to control Chagas disease by targeting mammalian host stages.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

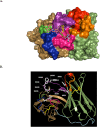

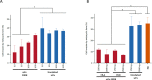
References
-
- Tibayrenc M, Barnabé C, Telleria J. Reticulate Evolution in: Medical and Epidemiological Implications In: Telleria J, Tibayrenc M, editors. American trypanosomiasis: Chagas disease One hundred years of research Burlington: Elsevier; 2010. 475–488.
-
- World Health Organization Health Topics, Chagas disease, 2017. www.who.int/topics/chagas_disease/en/. Accessed 04 Oct 2017
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

