Glucosamine Enhances TRAIL-Induced Apoptosis in the Prostate Cancer Cell Line DU145
- PMID: 31618900
- PMCID: PMC6963486
- DOI: 10.3390/medicines6040104
Glucosamine Enhances TRAIL-Induced Apoptosis in the Prostate Cancer Cell Line DU145
Abstract
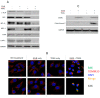
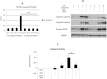
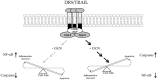
Background: Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) selectively kills tumor cells in cancer patients. However, patients often develop TRAIL resistance; thus, agents that can sensitize cells to TRAIL therapy would be beneficial clinically. Methods: Immunoblotting, flow cytometry, confocal microscopy, qPCR and caspase 8 activity assays were used to investigate whether glucosamine (GlcN) can sensitize cancer cells to TRAIL thereby enhancing apoptosis and potentially improving clinical response. Results: GlcN sensitized DU145 cells to TRAIL-induced apoptosis but did not increase death receptor 5 (DR5) cell surface expression. Once treated, these cells responded to TRAIL-induced apoptosis through both extrinsic and intrinsic apoptotic pathways as evidenced by the cleavage of both caspases 8 and 9. The combination of GlcN and TRAIL suppressed the expression of key anti-apoptotic factors cFLIP, BCL-XL, MCL-1 and XIAP and translocated BAK to the mitochondrial outer membrane thereby facilitating cytochrome C and SMAC release. In addition to the activation of apoptotic pathways, TRAIL-mediated inflammatory responses were attenuated by GlcN pretreatment reducing nuclear NF-kB levels and the expression of downstream target genes IL-6 and IL-8. Conclusions: GlcN/TRAIL combination could be a promising strategy for treating cancers by overcoming TRAIL resistance and abrogating TRAIL-induced inflammation.
Keywords: DR5; DU145; ER stress; TRAIL; apoptosis; cancer; glucosamine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

