Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger?
- PMID: 31618931
- PMCID: PMC6832215
- DOI: 10.3390/jcm8101690
Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger?
Abstract
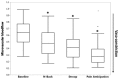
Sickle cell disease (SCD) is an inherited hemoglobinopathy characterized by polymerization of hemoglobin S upon deoxygenation that results in the formation of rigid sickled-shaped red blood cells that can occlude the microvasculature, which leads to sudden onsets of pain. The severity of vaso-occlusive crises (VOC) is quite variable among patients, which is not fully explained by their genetic and biological profiles. The mechanism that initiates the transition from steady state to VOC remains unknown, as is the role of clinically reported triggers such as stress, cold and pain. The rate of hemoglobin S polymerization after deoxygenation is an important determinant of vaso-occlusion. Similarly, the microvascular blood flow rate plays a critical role as fast-moving red blood cells are better able to escape the microvasculature before polymerization of deoxy-hemoglobin S causes the red cells to become rigid and lodge in small vessels. The role of the autonomic nervous system (ANS) activity in VOC initiation and propagation has been underestimated considering that the ANS is the major regulator of microvascular blood flow and that most triggers of VOC can alter the autonomic balance. Here, we will briefly review the evidence supporting the presence of ANS dysfunction in SCD, its implications in the onset of VOC, and how differences in autonomic vasoreactivity might potentially contribute to variability in VOC severity.
Keywords: autonomic nervous system dysfunction; microvascular blood flow; pain; sickle cell disease; vaso-occlusive crisis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

