A randomized, 29-day, dose-ranging, efficacy and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis
- PMID: 31619187
- PMCID: PMC6796426
- DOI: 10.1186/s12882-019-1547-z
A randomized, 29-day, dose-ranging, efficacy and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis
Abstract
Background: Daprodustat is a hypoxia-inducible factor-prolyl hydroxylase inhibitor currently being investigated as a treatment for anemia of chronic kidney disease (CKD) in both dialysis and nondialysis patients. In clinical studies to date, daprodustat has been administered orally as a once-daily regimen. This randomized, double-blind, placebo-controlled study characterized the initial dose-hemoglobin response as well as the efficacy and safety of three times weekly (TIW) daprodustat in hemodialysis patients switched from stable recombinant human erythropoietin (rhEPO), in accordance with a TIW hemodialysis schedule.
Methods: 103 patients on hemodialysis with baseline hemoglobin of 9.0 to 11.5 g/dL and previously receiving a stable dose of rhEPO or its analogs were randomized 1:1:1:1:1 to receive daprodustat 10, 15, 25, or 30 mg or placebo TIW over 29 days.
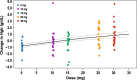
Results: Mean baseline hemoglobin was 10.6 g/dL for the placebo group and each daprodustat cohort. Daprodustat produced dose-dependent changes in mean hemoglobin from baseline to day 29. Using a Bayesian approach, the estimated dose conversion ratio between once-daily and TIW daprodustat was ~ 2.0 across the evaluated dose range using an Emax model. Daprodustat was generally well tolerated, with an adverse event (AE) profile consistent with the hemodialysis population.
Conclusions: These data help inform the appropriate dose conversion ratio to be applied to daily doses to obtain equivalent daprodustat TIW doses and suggest TIW treatment with daprodustat can treat anemia of CKD safely, supporting future long-term studies for this indication using a TIW dosing regimen.
Trial registration: ClinicalTrials.gov Identifier: NCT02689206 ; date registered: 02/11/2016.
Keywords: Anemia; Bayesian; Clinical trials; Dose response; Efficacy; Hemodialysis; Safety; Three-times weekly (TIW).
Conflict of interest statement
CKB, ARC, CH, KMM, and VVP are employees of and hold equity stock in GlaxoSmithKline (GSK). SC is a former employee of GSK and holds equity stock in the company.
Figures


References
-
- FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. Safety announcement issued June 24, 2011. http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm#sa. Accessed 12 Mar 2019.
-
- Kidney disease: improving global outcomes (KDIGO) anemia work group KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. doi: 10.1038/kisup.2012.37. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

