Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: the Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol
- PMID: 31619413
- PMCID: PMC6797426
- DOI: 10.1136/bmjopen-2018-025556
Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: the Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol
Abstract
Introduction: A broad range of stakeholders have called for randomised evidence on the potential clinical benefits and harms of proton therapy, a type of radiation therapy, for patients with breast cancer. Radiation therapy is an important component of curative treatment, reducing cancer recurrence and extending survival. Compared with photon therapy, the international treatment standard, proton therapy reduces incidental radiation to the heart. Our overall objective is to evaluate whether the differences between proton and photon therapy cardiac radiation dose distributions lead to meaningful reductions in cardiac morbidity and mortality after treatment for breast cancer.
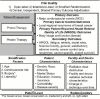
Methods: We are conducting a large scale, multicentre pragmatic randomised clinical trial for patients with breast cancer who will be followed longitudinally for cardiovascular morbidity and mortality, health-related quality of life and cancer control outcomes. A total of 1278 patients with non-metastatic breast cancer will be randomly allocated to receive either photon or proton therapy. The primary outcomes are major cardiovascular events, defined as myocardial infarction, coronary revascularisation, cardiovascular death or hospitalisation for unstable angina, heart failure, valvular disease, arrhythmia or pericardial disease. Secondary endpoints are urgent or unanticipated outpatient or emergency room visits for heart failure, arrhythmia, valvular disease or pericardial disease. The Radiotherapy Comparative Effectiveness (RadComp) Clinical Events Centre will conduct centralised, blinded adjudication of primary outcome events.
Ethics and dissemination: The RadComp trial has been approved by the institutional review boards of all participating sites. Recruitment began in February 2016. Current version of the protocol is A3, dated 08 November 2018. Dissemination plans include presentations at scientific conferences, scientific publications, stakeholder engagement efforts and presentation to the public via lay media outlets.
Trial registration number: NCT02603341.
Keywords: breast tumours; clinical trials; protocols & guidelines; radiation oncology.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JEB, SE, CDM and BK report grants from PCORI, during the conduct of the study. SNP, WB, L-MF, BGH, CK, MVM, RWM, FWP, SMM and OC report grants from University of Pennsylvania subcontracts as study investigator, during the conduct of the study.
Figures
References
-
- Ollendorf DA, Colby J. Proton beam therapy: ICER final evidence report Washington State Health Technology Assessment Program; 2014. http://www.hca.wa.gov/hta/Documents
-
- National Academies Initial national priorities for comparative effectiveness research: report brief, 2009. Available: https://www.nap.edu/read/12648/chapter/1 [Accessed 5 Sep 2017].
-
- Moya del Pina B, NCI Cancer Bulliten . Proton therapy for cancer: a new technology brief, 2009. Available: http://www.cancer.gov/ncicancerbulletin/090809/page8 [Accessed 12 Nov 2009].
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical