Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease
- PMID: 31619483
- PMCID: PMC6946483
- DOI: 10.1212/WNL.0000000000008441
Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease
Abstract
Objective: To determine the effects of 10 years of enzyme replacement therapy (ERT) in adult patients with Pompe disease, focusing on individual variability in treatment response.
Methods: In this prospective, multicenter cohort study, we studied 30 patients from the Netherlands and France who had started ERT during the only randomized placebo-controlled clinical trial with ERT in late-onset Pompe disease (NCT00158600) or its extension (NCT00455195) in 2005 to 2008. Main outcomes were walking ability (6-minute walk test [6MWT]), muscle strength (manual muscle testing using Medical Research Council [MRC] grading), and pulmonary function (forced vital capacity [FVC] in the upright and supine positions), assessed at 3- to 6-month intervals before and after the start of ERT. Data were analyzed with linear mixed-effects models for repeated measurements.
Results: Median follow-up duration on ERT was 9.8 years (interquartile range [IQR] 8.3-10.2 years). At the group level, baseline 6MWT was 49% of predicted (IQR 41%-60%) and had deteriorated by 22.2 percentage points (pp) at the 10-year treatment point (p < 0.001). Baseline FVC upright was 54% of predicted (IQR 47%-68%) and decreased by 11 pp over 10 years (p < 0.001). Effects of ERT on MRC sum score and FVC supine were similar. At the individual level, 93% of patients had initial benefit of ERT. Depending on the outcome measured, 35% to 63% of patients had a secondary decline after ≈3 to 5 years. Still, at 10 years of ERT, 52% had equal or better 6MWT and/or FVC upright compared to baseline.
Conclusions: The majority of patients with Pompe disease benefit from long-term ERT, but many patients experience some secondary decline after ≈3 to 5 years. Individual variation, however, is considerable.
Classification of evidence: This study provides Class IV evidence that for the majority of adults with Pompe disease, long-term ERT positively affects, or slows deterioration in, muscle strength, walking ability, and/or pulmonary function.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

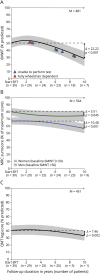


References
-
- van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet 2008;372:1342–1353. - PubMed
-
- Reuser AJJ, Hirschhorn R, Kroos MA. Pompe disease: glycogen storage disease type II, acid α-glucosidase (acid maltase) deficiency. In: Valle D, Beaudet AL, Vogelstein B, et al., editors. The Online Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2018;part 16, chapter 135.
-
- van der Beek NA, Hagemans ML, Reuser AJ, et al. . Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul Disord 2009;19:113–117. - PubMed
-
- Anderson LJ, Henley W, Wyatt KM, et al. . Effectiveness of enzyme replacement therapy in adults with late-onset Pompe disease: results from the NCS-LSD cohort study. J Inherit Metab Dis 2014;37:945–952. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
