Advanced Circuit and Cellular Imaging Methods in Nonhuman Primates
- PMID: 31619496
- PMCID: PMC6794937
- DOI: 10.1523/JNEUROSCI.1168-19.2019
Advanced Circuit and Cellular Imaging Methods in Nonhuman Primates
Abstract
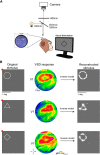
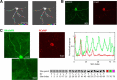
Novel genetically encoded tools and advanced microscopy methods have revolutionized neural circuit analyses in insects and rodents over the last two decades. Whereas numerous technical hurdles originally barred these methodologies from success in nonhuman primates (NHPs), current research has started to overcome those barriers. In some cases, methodological advances developed with NHPs have even surpassed their precursors. One such advance includes new ultra-large imaging windows on NHP cortex, which are larger than the entire rodent brain and allow analysis unprecedented ultra-large-scale circuits. NHP imaging chambers now remain patent for periods longer than a mouse's lifespan, allowing for long-term all-optical interrogation of identified circuits and neurons over timeframes that are relevant to human cognitive development. Here we present some recent imaging advances brought forth by research teams using macaques and marmosets. These include technical developments in optogenetics; voltage-, calcium- and glutamate-sensitive dye imaging; two-photon and wide-field optical imaging; viral delivery; and genetic expression of indicators and light-activated proteins that result in the visualization of tens of thousands of identified cortical neurons in NHPs. We describe a subset of the many recent advances in circuit and cellular imaging tools in NHPs focusing here primarily on the research presented during the corresponding mini-symposium at the 2019 Society for Neuroscience annual meeting.
Keywords: Adeno-Associated virus; cortical mapping; optogenetics; prosthetic vision; two-photon microscopy; voltage-sensitive dye imaging.
Copyright © 2019 the authors.
Figures


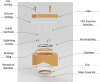
Cap and cap screws: removable cap for imaging and cleaning.
Silicone O-ring: ∼0.4 mm thick, prevents bacterial movement between chamber and cap.
Thin securing ring: Secures rotating ring against the bottom shelf of the chamber and prevents it from moving up.
Stabilizing screws: pushes against cup and prevents the imaging cup from moving closer to the chamber. Threaded into the bottom securing ring and penetrates silicone.
Guide cannulas: cannulas are threaded into the rotating ring at 3 different locations and sealed with silicone glue. These cannulas allow 18G CED needles to penetrate the cylinder.
CED injection needles: convection enhanced delivery needles. Designed for cortical injections but can also be used to deliver drugs or imaging contrast agents into the soft tissue.
Rotating ring: multiple threaded holes for the height adjusting screws and can rotate to adjust the positions of the screws. Sits between shelf in chamber and thin securing ring.
Chamber: Has holes for bone screws that are perpendicular to the surface of the bone to increase the strength of the bond between the chamber and bone. It also includes three threaded holes on the top to allow attachments and to secure the cap.
Resistance member: silicone is preferred for its ease in manufacturing, control of mechanical properties, and ability to be sterilized. Serves as a spring to adjust for pressure changes caused by the variations in swelling in the brain. Although not indicated in the drawing, the silicone connects to the lip of the imaging cup and rotating ring to create a sealed environment.
Imaging cup and glass cover slip: the glass is glued to the cup. Together they create a bowl that can hold liquid for a water-immersion objective.
References
-
- Adam Y, Kim JJ, Lou S, Zhao Y, Xie ME, Brinks D, Parot V, Wu H, Mostajo-Radji MA, Kheifets S, Parot V, Chettih S, Williams KJ, Gmeiner B, Farhi SL, Madisen L, Buchanan EK, Kinsella I, Zhou D, Paninski L, Harvey CD, Zeng H, et al. (2019) Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569:413–417. 10.1038/s41586-019-1166-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
