A TFAP2C Gene Signature Is Predictive of Outcome in HER2-Positive Breast Cancer
- PMID: 31619506
- PMCID: PMC6942205
- DOI: 10.1158/1541-7786.MCR-19-0359
A TFAP2C Gene Signature Is Predictive of Outcome in HER2-Positive Breast Cancer
Abstract
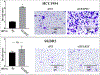
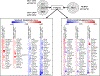
The AP-2γ transcription factor, encoded by the TFAP2C gene, regulates the expression of estrogen receptor-alpha (ERα) and other genes associated with hormone response in luminal breast cancer. Little is known about the role of AP-2γ in other breast cancer subtypes. A subset of HER2+ breast cancers with amplification of the TFAP2C gene locus becomes addicted to AP-2γ. Herein, we sought to define AP-2γ gene targets in HER2+ breast cancer and identify genes accounting for physiologic effects of growth and invasiveness regulated by AP-2γ. Comparing HER2+ cell lines that demonstrated differential response to growth and invasiveness with knockdown of TFAP2C, we identified a set of 68 differentially expressed target genes. CDH5 and CDKN1A were among the genes differentially regulated by AP-2γ and that contributed to growth and invasiveness. Pathway analysis implicated the MAPK13/p38δ and retinoic acid regulatory nodes, which were confirmed to display divergent responses in different HER2+ cancer lines. To confirm the clinical relevance of the genes identified, the AP-2γ gene signature was found to be highly predictive of outcome in patients with HER2+ breast cancer. We conclude that AP-2γ regulates a set of genes in HER2+ breast cancer that drive cancer growth and invasiveness. The AP-2γ gene signature predicts outcome of patients with HER2+ breast cancer and pathway analysis predicts that subsets of patients will respond to drugs that target the MAPK or retinoic acid pathways. IMPLICATIONS: A set of genes regulated by AP-2γ in HER2+ breast cancer that drive proliferation and invasion were identified and provided a gene signature that is predictive of outcome in HER2+ breast cancer.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures





References
-
- Weigel RJ, deConinck EC. Transcriptional control of estrogen receptor in estrogen receptor-negative breast carcinoma. Cancer research 1993;53(15):3472–4. - PubMed
-
- Woodfield GW, Horan AD, Chen Y, Weigel RJ. TFAP2C controls hormone response in breast cancer cells through multiple pathways of estrogen signaling. Cancer research 2007;67(18):8439–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

