Evaluation of OMNIgene Sputum and Ethanol Reagent for Preservation of Sputum Prior to Xpert and Culture Testing in Uganda
- PMID: 31619525
- PMCID: PMC6935912
- DOI: 10.1128/JCM.00810-19
Evaluation of OMNIgene Sputum and Ethanol Reagent for Preservation of Sputum Prior to Xpert and Culture Testing in Uganda
Abstract
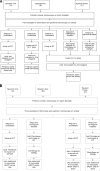
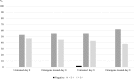
Xpert MTB/RIF (Xpert) and culture are the most reliable methods for tuberculosis diagnosis but are still poorly accessible in many low-resource countries. We aimed to assess the effects of OMNIgene Sputum (OM-S) and ethanol in preserving sputum for Xpert and OM-S for mycobacterial growth indicator tube (MGIT) testing over periods of 15 and 8 days, respectively. Sputum samples were collected from newly diagnosed smear-positive patients. For Xpert, pooled samples were split into 5 aliquots: 3 for Xpert on days 0, 7, and 15 without additive and 2 with either OM-S or ethanol at day 15. For MGIT, 2 aliquots were tested without preservative and 2 with OM-S at 0 and 8 days. Totals of 48 and 47 samples were included in the analysis for Xpert and culture. With Xpert, using day 0 as a reference, untreated samples stored for 7 and 15 days showed concordances of 45/46 (97.8%) and 46/48 (95.8%). For samples preserved with OM-S or ethanol for 15 days compared with untreated samples processed at day 0 or after 15 days, OM-S concordances were 46/48 (95.8%) and 47/48 (97.9%), while those of ethanol were 44/48 (91.7%) and 45/48 (93.8%). With MGIT, concordances between untreated and OM-S-treated samples were 21/41 (51.2%) at day 0 and 21/44 (47.7%) at day 8. In conclusion, Xpert equally detected tuberculosis in OM-S-treated and untreated samples up to 15 days but showed slightly lower detection in ethanol-treated samples. Among OM-S-treated samples, MGIT positivity was significantly lower than in untreated samples at both time points.
Keywords: OMNIgene; Xpert; culture; tuberculosis.
Copyright © 2019 American Society for Microbiology.
Figures
References
-
- WHO. 2017. Global tuberculosis report 2018. Pharmacol Rep 69:683–690. - PubMed
-
- WHO. 2014. Xpert MTB/RIF implementation manual: technical and operational ‘how-to’: practical considerations. WHO, Geneva, Switzerland. - PubMed
-
- WHO. 2011. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF. Policy statement. WHO, Geneva, Switzerland. - PubMed
-
- Cepheid. 2009. Operator manual test Xpert MTB/RIF. Cepheid, Sunnyvale, CA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical