Alveolar macrophage secretion of vesicular SOCS3 represents a platform for lung cancer therapeutics
- PMID: 31619584
- PMCID: PMC6824301
- DOI: 10.1172/jci.insight.131340
Alveolar macrophage secretion of vesicular SOCS3 represents a platform for lung cancer therapeutics
Abstract
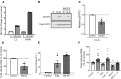
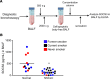
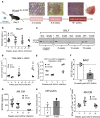
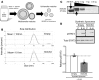
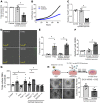
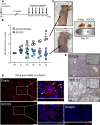
Lung cancer remains the leading cause of cancer-related death in the United States. Although the alveolar macrophage (AM) comprises the major resident immune cell in the lung, few studies have investigated its role in lung cancer development. We recently discovered a potentially novel mechanism wherein AMs regulate STAT-induced inflammatory responses in neighboring epithelial cells (ECs) via secretion and delivery of suppressors of cytokine signaling 3 (SOCS3) within extracellular vesicles (EVs). Here, we explored the impact of SOCS3 transfer on EC tumorigenesis and the integrity of AM SOCS3 secretion during development of lung cancer. AM-derived EVs containing SOCS3 inhibited STAT3 activation as well as proliferation and survival of lung adenocarcinoma cells. Levels of secreted SOCS3 were diminished in lungs of patients with non-small cell lung cancer and in a mouse model of lung cancer, and the impaired ability of murine AMs to secrete SOCS3 within EVs preceded the development of lung tumors. Loss of this homeostatic brake on tumorigenesis prompted our effort to "rescue" it. Provision of recombinant SOCS3 loaded within synthetic liposomes inhibited proliferation and survival of lung adenocarcinoma cells in vitro as well as malignant transformation of normal ECs. Intratumoral injection of SOCS3 liposomes attenuated tumor growth in a lung cancer xenograft model. This work identifies AM-derived vesicular SOCS3 as an endogenous antitumor mechanism that is disrupted within the tumor microenvironment and whose rescue by synthetic liposomes can be leveraged as a potential therapeutic strategy for lung cancer.
Keywords: Cancer; Immunology; Macrophages; Oncology; Tumor suppressors.
Conflict of interest statement
Figures
References
-
- Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67(18):8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

