STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice
- PMID: 31619589
- PMCID: PMC6824303
- DOI: 10.1172/jci.insight.131355
STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice
Abstract
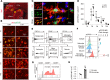
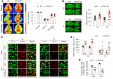
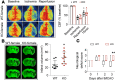
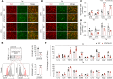
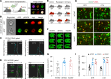
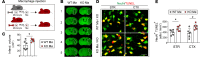
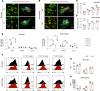
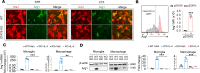
Efferocytosis, or phagocytic clearance of dead/dying cells by brain-resident microglia and/or infiltrating macrophages, is instrumental for inflammation resolution and restoration of brain homeostasis after stroke. Here, we identify the signal transducer and activator of transcription 6/arginase1 (STAT6/Arg1) signaling axis as a potentially novel mechanism that orchestrates microglia/macrophage responses in the ischemic brain. Activation of STAT6 was observed in microglia/macrophages in the ischemic territory in a mouse model of stroke and in stroke patients. STAT6 deficiency resulted in reduced clearance of dead/dying neurons, increased inflammatory gene signature in microglia/macrophages, and enlarged infarct volume early after experimental stroke. All of these pathological changes culminated in an increased brain tissue loss and exacerbated long-term functional deficits. Combined in vivo analyses using BM chimeras and in vitro experiments using microglia/macrophage-neuron cocultures confirmed that STAT6 activation in both microglia and macrophages was essential for neuroprotection. Adoptive transfer of WT macrophages into STAT6-KO mice reduced accumulation of dead neurons in the ischemic territory and ameliorated brain infarction. Furthermore, decreased expression of Arg1 in STAT6-/- microglia/macrophages was responsible for impairments in efferocytosis and loss of antiinflammatory modality. Our study suggests that efferocytosis via STAT6/Arg1 modulates microglia/macrophage phenotype, accelerates inflammation resolution, and improves stroke outcomes.
Keywords: Inflammation; Neuroscience; Stroke.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

