Strategies for the Identification of Bioactive Neuropeptides in Vertebrates
- PMID: 31619945
- PMCID: PMC6759750
- DOI: 10.3389/fnins.2019.00948
Strategies for the Identification of Bioactive Neuropeptides in Vertebrates
Abstract
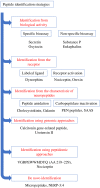
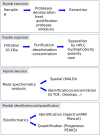
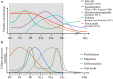
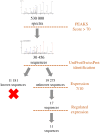
Neuropeptides exert essential functions in animal physiology by controlling e.g., reproduction, development, growth, energy homeostasis, cardiovascular activity and stress response. Thus, identification of neuropeptides has been a very active field of research over the last decades. This review article presents the various methods used to discover novel bioactive peptides in vertebrates. Initially identified on the basis of their biological activity, some neuropeptides have also been discovered for their ability to bind/activate a specific receptor or based on their biochemical characteristics such as C-terminal amidation which concerns half of the known neuropeptides. More recently, sequencing of the genome of many representative species has facilitated peptidomic approaches using mass spectrometry and in silico screening of genomic libraries. Through these different approaches, more than a hundred of bioactive neuropeptides have already been identified in vertebrates. Nevertheless, researchers continue to find new neuropeptides or to identify novel functions of neuropeptides that had not been detected previously, as it was recently the case for nociceptin.
Keywords: bioactiity; de novo; identification; neuropeptide; peptidomic approach; review.
Copyright © 2019 Corbière, Vaudry, Chan, Walet-Balieu, Lecroq, Lefebvre, Pineau and Vaudry.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

