JNK Signaling Pathway Mediates Acetaminophen-Induced Hepatotoxicity Accompanied by Changes of Glutathione S-Transferase A1 Content and Expression
- PMID: 31620005
- PMCID: PMC6763582
- DOI: 10.3389/fphar.2019.01092
JNK Signaling Pathway Mediates Acetaminophen-Induced Hepatotoxicity Accompanied by Changes of Glutathione S-Transferase A1 Content and Expression
Abstract
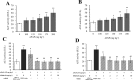
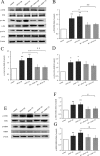
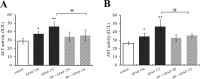
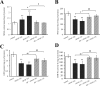
Acetaminophen (APAP) is an analgesic-antipyretic drug and widely used in clinics. Its overdose may cause serious liver damage. Here, we examined the mechanistic role of c-Jun N-terminal kinase (JNK) signaling pathway in liver injury induced by different doses of APAP. Male mice were treated with APAP (150 and 175 mg·kg-1), and meanwhile JNK inhibitor SP600125 was used to interfere APAP-induced liver damage. The results showed that JNK signaling pathway was activated by APAP in a dose-dependent manner. C-Jun N-terminal kinase inhibitor decreased JNK and c-Jun activation significantly (P < 0.01) at 175 mg·kg-1 APAP dose, and phosphorylation levels of upstream proteins of JNK were also decreased markedly (P < 0.05). In addition, serum aminotransferases activities and hepatic oxidative stress increased in a dose-dependent manner with APAP treatment, but the levels of aminotransferases and oxidative stress decreased in mice treated with JNK inhibitor, which implied that JNK inhibition ameliorated APAP-induced liver damage. It was observed that apoptosis was increased in APAP-induced liver injury, and SP600125 can attenuate apoptosis through the inhibition of JNK phosphorylation. Meanwhile, glutathione S-transferases A1 (GSTA1) content in serum was enhanced, while GSTA1 content and expression in liver reduced significantly with administration of APAP (150 and 175 mg·kg-1). After inhibiting JNK, GSTA1 content in serum decreased significantly (P < 0.01); meanwhile, GSTA1 content and expression in liver enhanced. These findings suggested that JNK signaling pathway mediated APAP-induced hepatic injury, which was accompanied by varying GSTA1 content and expression in liver and serum.
Keywords: acetaminophen; c-Jun N-terminal kinase; glutathione S-transferases A1; hepatotoxicity; liver injury.
Copyright © 2019 Shi, Hao, Yang, Muhammad, Zhang, Chang, Li, Li, Li and Liu.
Figures


Similar articles
-
Hydrogen sulfide protects against acetaminophen-induced acute liver injury by inhibiting apoptosis via the JNK/MAPK signaling pathway.J Cell Biochem. 2019 Mar;120(3):4385-4397. doi: 10.1002/jcb.27724. Epub 2018 Sep 27. J Cell Biochem. 2019. PMID: 30260040
-
Protection afforded by pre- or post-treatment with 4-phenylbutyrate against liver injury induced by acetaminophen overdose in mice.Pharmacol Res. 2014 Sep;87:26-41. doi: 10.1016/j.phrs.2014.06.003. Epub 2014 Jun 18. Pharmacol Res. 2014. PMID: 24951965
-
Resistance to acetaminophen-induced hepatotoxicity in glutathione S-transferase Mu 1-null mice.J Toxicol Sci. 2012;37(3):595-605. doi: 10.2131/jts.37.595. J Toxicol Sci. 2012. PMID: 22687999
-
Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice.Int Immunopharmacol. 2017 Apr;45:26-33. doi: 10.1016/j.intimp.2017.01.028. Epub 2017 Jan 31. Int Immunopharmacol. 2017. PMID: 28152447
-
[c-Jun N-terminal kinase signaling pathway in acetaminophen-induced liver injury].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023 Nov;35(11):1223-1228. doi: 10.3760/cma.j.cn121430-20221205-01060. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023. PMID: 37987136 Review. Chinese.
Cited by
-
Hepatoprotective Effect of Oplopanax elatus Nakai Adventitious Roots Extract by Regulating CYP450 and PPAR Signaling Pathway.Front Pharmacol. 2022 May 2;13:761618. doi: 10.3389/fphar.2022.761618. eCollection 2022. Front Pharmacol. 2022. PMID: 35586046 Free PMC article.
-
Evaluation of the protective effect of losartan in acetaminophen-induced liver and kidney damage in mice.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):5067-5078. doi: 10.1007/s00210-023-02937-0. Epub 2024 Jan 9. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38194107 Free PMC article.
-
The Late-Stage Protective Effect of Mito-TEMPO against Acetaminophen-Induced Hepatotoxicity in Mouse and Three-Dimensional Cell Culture Models.Antioxidants (Basel). 2020 Oct 9;9(10):965. doi: 10.3390/antiox9100965. Antioxidants (Basel). 2020. PMID: 33050213 Free PMC article.
-
Hepatoprotective effect of kaempferol glycosides isolated from Cedrela odorata L. leaves in albino mice: involvement of Raf/MAPK pathway.Res Pharm Sci. 2021 Jun 30;16(4):370-380. doi: 10.4103/1735-5362.319575. eCollection 2021 Aug. Res Pharm Sci. 2021. PMID: 34447445 Free PMC article.
-
The hepatoprotective candidates by synergistic formula of marine and terrestrial against Acetaminophen toxicity using in-vitro, in-vivo, and in silico screening approach.Saudi J Biol Sci. 2023 Apr;30(4):103607. doi: 10.1016/j.sjbs.2023.103607. Epub 2023 Feb 24. Saudi J Biol Sci. 2023. PMID: 36941882 Free PMC article.
References
-
- Abdel-Zaher A. O., Abdel-Rahman M. M., Hafez M. M., Omran F. M. (2007). Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology 234 (1), 124–134. 10.1016/j.tox.2007.02.014 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

